Critical role for tumor necrosis factor alpha in controlling the number of lumenal pathogenic bacteria and immunopathology in infectious colitis - PubMed (original) (raw)
Critical role for tumor necrosis factor alpha in controlling the number of lumenal pathogenic bacteria and immunopathology in infectious colitis
N S Gonçalves et al. Infect Immun. 2001 Nov.
Abstract
Infection of mice with the intestinal bacterial pathogen Citrobacter rodentium results in colonic mucosal hyperplasia and a local Th1 inflammatory response similar to that seen in mouse models of inflammatory bowel disease. In these latter models, and in patients with Crohn's disease, neutralization of tumor necrosis factor alpha (TNF-alpha) is of therapeutic benefit. Since there is no information on the role of TNF-alpha in either immunity to noninvasive bacterial pathogens or on the role of TNF-alpha in the immunopathology of infectious colitis, we investigated C. rodentium infection in TNFRp55(-/-) mice. In TNFRp55(-/-) mice, there were higher colonic bacterial burdens, but the organisms were cleared at the same rate as C57BL/6 mice, showing that TNF-alpha is not needed for protective antibacterial immunity. The most striking feature of infection in TNFRp55(-/-) mice, however, was the markedly enhanced pathology, with increased mucosal weight and thickness, increased T-cell infiltrate, and a markedly greater mucosal Th1 response. Interleukin-12 p40 transcripts were markedly elevated in C. rodentium-infected TNFRp55(-/-) mice, and this was associated with enhanced mucosal STAT4 phosphorylation. TNF-alpha is not obligatory for protective immunity to C. rodentium in mice; however, it appears to play some role in downregulating mucosal pathology and Th1 immune responses.
Figures
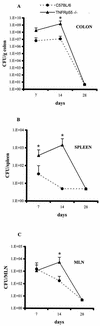
FIG. 1
Bacterial load in _C. rodentium_-infected C57BL/6 and TNFRp55−/− mice. Bacterial counts were determined in the colon (A), spleen (B), and MLN (C) at the indicated time points. Each point reflects the means and SEM (error bars) of five mice per group (✽, P < 0.05). The data shown are from one experiment of three, which yielded identical results. The lower limit detection of the bacteria is 10 CFU per organ.
FIG. 2
Intimin staining of _C. rodentium_-infected C57BL/6 mice (magnifications: A, ×100; C, ×1,000; F, ×400) and TNFRp55−/− mice (magnifications: B, ×100; D, ×1,000). (D) Bacteria can be seen along the surface epithelium in both groups of mice (arrows) and occasionally deep in the glands in the infected TNFRp55−/− mice. (E) Rabbit isotype control IgG showed no staining (magnification, ×400). Another feature was that the anti-intimin antibody, in addition to staining the bacteria, also stained epithelial cells underlying areas of bacterial colonization (D, large arrows).
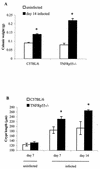
FIG. 3
Weight of the distal colon (A) and crypt length (B) in uninfected and _C. rodentium_-infected C57BL/6 and TNFRp55−/− mice on days 7 and 14. Each group contained five mice (✽, P < 0.05).
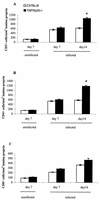
FIG. 4
Cell counts for CD3+ (A), CD4+ (B), and CD8+ (C) cells infiltrating the lamina propria in uninfected and _C. rodentium_-infected C57BL/6 and TNFRp55−/− mice on days 7 and 14. Each group represents five mice (the mean ± SEM) (✽, P < 0.05).
FIG. 5
Cytokine mRNA transcripts in gut tissue of uninfected and _C. rodentium_-infected C57BL/6 and TNFRp55−/− mice on days 7 and 14. Each group represents five mice (the mean ± SEM; ✽, P < 0.05). (A) IFN-γ. (B) IL-12p40. (C) TNF-α. (D) IL-4.
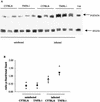
FIG. 6
Western blot analysis of Stat4 and tyrosine-phosphorylated Stat4 in the gut tissue of uninfected and _C. rodentium_-infected C57BL/6 and TNFRp55−/− mice on day 14. Three mice per group were analyzed in each of two independent experiments, and representative autoradiograph is shown in panel A. An increase in p-Stat4 expression was seen in the infected TNFRp55−/− mice at day 14. (B) The density of the bands was also quantified. In total, we analyzed 5 to 6 mice per group. As positive control (+ve), we used proteins extracted from human peripheral blood mononuclear cells preactivated with PHA and then stimulated with IL-12, the major Stat4-inducing cytokine.
Similar articles
- Impaired immunity to intestinal bacterial infection in stromelysin-1 (matrix metalloproteinase-3)-deficient mice.
Li CK, Pender SL, Pickard KM, Chance V, Holloway JA, Huett A, Gonçalves NS, Mudgett JS, Dougan G, Frankel G, MacDonald TT. Li CK, et al. J Immunol. 2004 Oct 15;173(8):5171-9. doi: 10.4049/jimmunol.173.8.5171. J Immunol. 2004. PMID: 15470062 - Citrobacter rodentium-induced colitis: A robust model to study mucosal immune responses in the gut.
Koroleva EP, Halperin S, Gubernatorova EO, Macho-Fernandez E, Spencer CM, Tumanov AV. Koroleva EP, et al. J Immunol Methods. 2015 Jun;421:61-72. doi: 10.1016/j.jim.2015.02.003. Epub 2015 Feb 19. J Immunol Methods. 2015. PMID: 25702536 - Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium.
Simmons CP, Clare S, Ghaem-Maghami M, Uren TK, Rankin J, Huett A, Goldin R, Lewis DJ, MacDonald TT, Strugnell RA, Frankel G, Dougan G. Simmons CP, et al. Infect Immun. 2003 Sep;71(9):5077-86. doi: 10.1128/IAI.71.9.5077-5086.2003. Infect Immun. 2003. PMID: 12933850 Free PMC article. - Host defences to Citrobacter rodentium.
MacDonald TT, Frankel G, Dougan G, Goncalves NS, Simmons C. MacDonald TT, et al. Int J Med Microbiol. 2003 Apr;293(1):87-93. doi: 10.1078/1438-4221-00247. Int J Med Microbiol. 2003. PMID: 12755369 Review. - Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia.
Luperchio SA, Schauer DB. Luperchio SA, et al. Microbes Infect. 2001 Apr;3(4):333-40. doi: 10.1016/s1286-4579(01)01387-9. Microbes Infect. 2001. PMID: 11334751 Review.
Cited by
- Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis.
Weng M, Huntley D, Huang IF, Foye-Jackson O, Wang L, Sarkissian A, Zhou Q, Walker WA, Cherayil BJ, Shi HN. Weng M, et al. J Immunol. 2007 Oct 1;179(7):4721-31. doi: 10.4049/jimmunol.179.7.4721. J Immunol. 2007. PMID: 17878371 Free PMC article. - Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice.
Khan MA, Ma C, Knodler LA, Valdez Y, Rosenberger CM, Deng W, Finlay BB, Vallance BA. Khan MA, et al. Infect Immun. 2006 May;74(5):2522-36. doi: 10.1128/IAI.74.5.2522-2536.2006. Infect Immun. 2006. PMID: 16622187 Free PMC article. - Current perspective on biological properties of plasmacytoid dendritic cells and dysfunction in gut.
Guo X, He C, Xin S, Gao H, Wang B, Liu X, Zhang S, Gong F, Yu X, Pan L, Sun F, Xu J. Guo X, et al. Immun Inflamm Dis. 2023 Sep;11(9):e1005. doi: 10.1002/iid3.1005. Immun Inflamm Dis. 2023. PMID: 37773693 Free PMC article. Review. - HVEM: An unusual TNF receptor family member important for mucosal innate immune responses to microbes.
Shui JW, Kronenberg M. Shui JW, et al. Gut Microbes. 2013 Mar-Apr;4(2):146-51. doi: 10.4161/gmic.23443. Epub 2013 Jan 18. Gut Microbes. 2013. PMID: 23333859 Free PMC article. Review. - Chitinase 3-like-1 promotes Streptococcus pneumoniae killing and augments host tolerance to lung antibacterial responses.
Dela Cruz CS, Liu W, He CH, Jacoby A, Gornitzky A, Ma B, Flavell R, Lee CG, Elias JA. Dela Cruz CS, et al. Cell Host Microbe. 2012 Jul 19;12(1):34-46. doi: 10.1016/j.chom.2012.05.017. Cell Host Microbe. 2012. PMID: 22817986 Free PMC article.
References
- Barthold S W. The microbiology of transmissible murine colonic hyperplasia. Lab Anim Sci. 1980;30:167–173. - PubMed
- Binion D G, West G A, Ina K, Ziats N P, Emancipator S N, Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology. 1997;112:1895–1907. - PubMed
- Chedid M, Rubin J S, Csaky K G, Aaronson S A. Regulation of keratinocyte growth factor gene expression by interleukin 1. J Biol Chem. 1994;269:10753–10757. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous