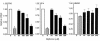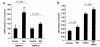Role of AMP-activated protein kinase in mechanism of metformin action - PubMed (original) (raw)
. 2001 Oct;108(8):1167-74.
doi: 10.1172/JCI13505.
R Myers, Y Li, Y Chen, X Shen, J Fenyk-Melody, M Wu, J Ventre, T Doebber, N Fujii, N Musi, M F Hirshman, L J Goodyear, D E Moller
Affiliations
- PMID: 11602624
- PMCID: PMC209533
- DOI: 10.1172/JCI13505
Role of AMP-activated protein kinase in mechanism of metformin action
G Zhou et al. J Clin Invest. 2001 Oct.
Abstract
Metformin is a widely used drug for treatment of type 2 diabetes with no defined cellular mechanism of action. Its glucose-lowering effect results from decreased hepatic glucose production and increased glucose utilization. Metformin's beneficial effects on circulating lipids have been linked to reduced fatty liver. AMP-activated protein kinase (AMPK) is a major cellular regulator of lipid and glucose metabolism. Here we report that metformin activates AMPK in hepatocytes; as a result, acetyl-CoA carboxylase (ACC) activity is reduced, fatty acid oxidation is induced, and expression of lipogenic enzymes is suppressed. Activation of AMPK by metformin or an adenosine analogue suppresses expression of SREBP-1, a key lipogenic transcription factor. In metformin-treated rats, hepatic expression of SREBP-1 (and other lipogenic) mRNAs and protein is reduced; activity of the AMPK target, ACC, is also reduced. Using a novel AMPK inhibitor, we find that AMPK activation is required for metformin's inhibitory effect on glucose production by hepatocytes. In isolated rat skeletal muscles, metformin stimulates glucose uptake coincident with AMPK activation. Activation of AMPK provides a unified explanation for the pleiotropic beneficial effects of this drug; these results also suggest that alternative means of modulating AMPK should be useful for the treatment of metabolic disorders.
Figures
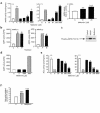
Figure 1
Metformin mediates AMPK activation in primary hepatocytes. (a) Metformin (black bars) and AICAR (A; 500 μM) activate AMPK in rat primary hepatocytes. The treatments were 1 hour, 7 hours, and 39 hours, respectively. (b) Metformin (500 μM) and AICAR (500 μM) activated both AMPKα1 and AMPKα2 complexes demonstrated by immunoprecipitation-AMPK assay. DPM, disintegrations per minute. (c) Metformin (1 mM) and AICAR (500 μM) stimulated AMPK Thr172 phosphorylation. (d) Metformin does not activate partially purified rat liver AMPK in vitro. (e) Metformin and AICAR (500 μM) inactivate ACC in rat primary hepatocytes. (f) Metformin (500 μM, 4 hours) and AICAR (500 μM, 4 hours) stimulate hepatocyte fatty acid oxidation. C, vehicle control. Mean (n = 3 wells per treatment for 1 hour and 7 hours; for 39-hour treatment, n = 12–15 wells per treatment) ± SEM values are shown. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control medium (paired t test).
Figure 2
Treatment of hepatocytes with metformin or AICAR suppresses lipogenic genes in rat primary hepatocytes. Taqman-based real-time RT-PCR was used to quantitate mRNAs for FAS, S14, and HMGR. Mean (n = 3 per treatment) ± SEM values are shown. *P < 0.05, **P < 0.01 vs. control medium (paired t test). C, control; A, positive control (AICAR, 500 μM). These experiments were repeated a minimum of three times with similar results.
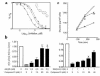
Figure 3
Discovery and use of a novel small-molecule AMPK inhibitor to establish that the effects of metformin on ACC and glucose production are AMPK dependent. (a) Inhibition of partially purified AMPK by compound C (inset shows the chemical structure) is reversible and competitive with respect to ATP. The in vitro kinase assay was performed in the presence of 5 μM ATP, 0 μM AMP (filled circles), 100 μM ATP, 0 μM AMP (filled squares), and 100 μM ATP plus 100 μM AMP (open circles). (b) Compound C inhibits the effects of AICAR and metformin on ACC in rat hepatocytes. Mean (n = 3 per treatment) ± SEM values are shown. *P < 0.05 vs. control medium (paired t test). (c) Compound C attenuates the ability of metformin to suppress glucagon-stimulated glucose production by hepatocytes from 24-hour starved rats. At time 0, compound C (40 μM) was added, and at time 30 minutes, metformin (2 mM) was added. Squares, control; diamonds, metformin; circles, metformin plus compound C. Each point represents the mean ± SEM of four replicate assays. *P < 0.05, **P < 0.01 vs. metformin alone at the same time point (paired t test). The experiment was repeated three times with similar results.
Figure 4
Metformin (Met; 2 mM, 3 hours) stimulates AMPK activity in skeletal muscle in association with induction of glucose uptake. (a) AMPK activity; (b) glucose uptake. Mean (n = 5–6 isolated rat epitrochlearis muscles per treatment) ± SEM values is shown.
Figure 5
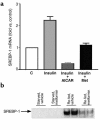
Metformin and AICAR downregulate hepatic SREBP-1. (a) Metformin (500 μM) and AICAR (500 μM) have similar effects to suppress SREBP-1 mRNA expression in rat hepatocytes. Mean ± SEM (n = 3 replicate assays) are shown. Insulin significantly increased SREBP-1 mRNA (P = 0.03 vs. control medium by Student’s t test). Both AICAR and metformin significantly decreased SREBP-1 mRNA with P values of 0.0046 and 0.01, respectively, versus insulin. (b) Metformin prevents re-fed stimulated accumulation of mature SREBP-1 in nuclear extracts from treated rats. Western blot analysis using a monoclonal anti–SREBP-1 Ab (prepared from hybridoma CRL-2121) was performed. Similar results were obtained from a separate in vivo experiment.
Figure 6
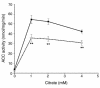
Metformin inhibits hepatic ACC activity in vivo. ACC activity was analyzed in the presence or absence of the indicated citrate concentrations using liver samples from fed rats treated for 5 days with control vehicle (filled squares, n = 7) or treated with 200 mg/kg (twice daily oral gavage) metformin (open squares,n = 8). **P < 0.01.
Figure 7
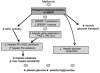
Model for the mechanism by which metformin mediates effects on lipid and glucose metabolism. FA, fatty acid.
Comment in
- The blooming of the French lilac.
Witters LA. Witters LA. J Clin Invest. 2001 Oct;108(8):1105-7. doi: 10.1172/JCI14178. J Clin Invest. 2001. PMID: 11602616 Free PMC article. Review. No abstract available.
Similar articles
- AMP-activated protein kinase phosphorylates transcription factors of the CREB family.
Thomson DM, Herway ST, Fillmore N, Kim H, Brown JD, Barrow JR, Winder WW. Thomson DM, et al. J Appl Physiol (1985). 2008 Feb;104(2):429-38. doi: 10.1152/japplphysiol.00900.2007. Epub 2007 Dec 6. J Appl Physiol (1985). 2008. PMID: 18063805 - Control of p70 ribosomal protein S6 kinase and acetyl-CoA carboxylase by AMP-activated protein kinase and protein phosphatases in isolated hepatocytes.
Krause U, Bertrand L, Hue L. Krause U, et al. Eur J Biochem. 2002 Aug;269(15):3751-9. doi: 10.1046/j.1432-1033.2002.03074.x. Eur J Biochem. 2002. PMID: 12153572 - AMP-activated protein kinase and muscle glucose uptake.
Musi N, Goodyear LJ. Musi N, et al. Acta Physiol Scand. 2003 Aug;178(4):337-45. doi: 10.1046/j.1365-201X.2003.01168.x. Acta Physiol Scand. 2003. PMID: 12864738 Review. - Targeting the AMP-activated protein kinase for the treatment of type 2 diabetes.
Musi N, Goodyear LJ. Musi N, et al. Curr Drug Targets Immune Endocr Metabol Disord. 2002 Jul;2(2):119-27. Curr Drug Targets Immune Endocr Metabol Disord. 2002. PMID: 12476786 Review.
Cited by
- Glucose transporters in the small intestine in health and disease.
Koepsell H. Koepsell H. Pflugers Arch. 2020 Sep;472(9):1207-1248. doi: 10.1007/s00424-020-02439-5. Epub 2020 Aug 23. Pflugers Arch. 2020. PMID: 32829466 Free PMC article. Review. - CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction.
Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW. Peterson JM, et al. Am J Physiol Regul Integr Comp Physiol. 2013 Sep;305(5):R522-33. doi: 10.1152/ajpregu.00110.2013. Epub 2013 Jul 10. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23842676 Free PMC article. - The genetic advantage of healthy centenarians: unraveling the central role of NLRP3 in exceptional healthspan.
Verlinden SF. Verlinden SF. Front Aging. 2024 Sep 5;5:1452453. doi: 10.3389/fragi.2024.1452453. eCollection 2024. Front Aging. 2024. PMID: 39301197 Free PMC article. Review. - Synergistic effects of caffeine and catechins on lipid metabolism in chronically fed mice via the AMP-activated protein kinase signaling pathway.
Zhao Y, Yang L, Huang Z, Lin L, Zheng G. Zhao Y, et al. Eur J Nutr. 2017 Oct;56(7):2309-2318. doi: 10.1007/s00394-016-1271-4. Epub 2016 Jul 21. Eur J Nutr. 2017. PMID: 27444711 - Clinical Approach to Vascular Calcification in Patients With Non-dialysis Dependent Chronic Kidney Disease: Mineral-Bone Disorder-Related Aspects.
Bover J, Aguilar A, Arana C, Molina P, Lloret MJ, Ochoa J, Berná G, Gutiérrez-Maza YG, Rodrigues N, D'Marco L, Górriz JL. Bover J, et al. Front Med (Lausanne). 2021 May 19;8:642718. doi: 10.3389/fmed.2021.642718. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34095165 Free PMC article. Review.
References
- 2000. IMS HEALTH’s National Prescription Audit PlusTM. http://www.ims-global.com.
- Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. - PubMed
- Wiernsperger NF, Bailey CJ. The antihyperglycaemic effect of metformin: therapeutic and cellular mechanisms. Drugs. 1999;58:31–39. - PubMed
- Wu MS, et al. Effect of metformin on carbohydrate and lipoprotein metabolism in NIDDM patients. Diabetes Care. 1990;13:1–8. - PubMed
- Schafer G. Biguanides. A review of history, pharmacodynamics and therapy. Diabete Metab. 1983;9:148–163. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases