Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance - PubMed (original) (raw)
Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance
C Guerra et al. J Clin Invest. 2001 Oct.
Expression of concern in
- Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance.
Guerra C, Navarro P, Valverde AM, Arribas M, Brüning J, Kozak LP, Kahn CR, Benito M. Guerra C, et al. J Clin Invest. 2019 Jan 2;129(1):437. doi: 10.1172/JCI126191. Epub 2019 Jan 2. J Clin Invest. 2019. PMID: 30601142 Free PMC article. No abstract available.
Abstract
Although insulin regulates metabolism in both brown and white adipocytes, the role of these tissues in energy storage and utilization is quite different. Recombination technology using the Cre-loxP approach allows inactivation of the insulin receptor in a tissue-specific manner. Mice lacking insulin receptors in brown adipocytes show an age-dependent loss of interscapular brown fat but increased expression of uncoupling protein-1 and -2. In parallel, these mice develop an insulin-secretion defect resulting in a progressive glucose intolerance, without insulin resistance. This model provides direct evidence for not only a role for the insulin receptors in brown fat adipogenesis, the data also suggest a novel role of brown adipose tissue in the regulation of insulin secretion and glucose homeostasis.
Figures
Figure 1
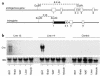
Structure and expression of the UCP-1-Cre transgene. (a) The Cre cDNA sequence was included at the BglII site of the first exon of UCP-1 gene. The length of 5′ UTR of the UCP-1 gene was 8.4 kb. Exons 3, 4, 5, and 6 of the UCP-1 gene were also incorporated downstream of the Cre cDNA sequence. (b) Representative Northern blot analysis of total RNA isolated from several tissues of the two transgenic lines (lines 13 and 14) of mice carrying a UCP-1-Cre DNA construct described in a and C57BL/6J (B6) control mouse. Blots were hybridized with a full-length Cre cDNA probe.
Figure 2
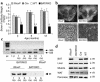
BAT content and IR expression. Interscapular brown fat was obtained from WT, Cre, IR_lox_P, and BATIRKO mice at 3, 6, and 12 months of age. (a) BAT weight vs. body weight is represented. Results are expressed as a mean ± SEM (n = 10–20). #P < 0.00001, BATIRKO vs. IR_lox_P; **P < 0.0001, BATIRKO vs. IR_lox_P or Cre; *P < 0.01, BATIRKO vs. IR_lox_P or Cre or WT. Representative interscapular BAT from IR_lox_P and BATIRKO mice at 6 months of age (b) (upper panel). Hematoxylin and eosin stain of BAT from IR_lox_P and BATIRKO mice at 6 months of age (b) (lower panel). RT-PCR analysis of RNA prepared from BAT from BATIRKO mice at different ages (1 day and 1, 3, 6, and 12 months) and IR_lox_P at 6 months of age, to study IR expression. The RT reaction was carried out as described in Methods. A larger band (585 bp) was observed in the IR_lox_P lane, while a smaller band of 435 bp was observed in BATIRKO mice lanes, suggesting a recombination event (c) (upper panel). Protein extracts of BAT from IR_lox_P and BATIRKO mice at different ages (1, 3, and 6 months) were subjected to immunoprecipitation with an mAb against IR β-chain and analyzed by Western blot. This is representative of three experiments (c) (lower panel). Protein extracts from different tissues of 6-month-old WT, IR_loxP_, Cre, and BATIRKO mice were immunoprecipitated with the same Ab and analyzed by Western blot. This is representative of three experiments (d). φx, DNA marker.
Figure 3
Gene expression throughout development. Skeletal muscle (Muscle), interscapular BAT, and WAT were obtained at 3 (a) (upper panel), 6 (b) (upper panel), and 12 months (c) (upper panel) of age from IR_lox_P, Cre, and BATIRKO mice. Total RNA was submitted to Northern blot analysis and hybridized with labeled UCP-1, UCP-2, ME, FAS, G3PD, PPARγ, C/EBP-α, or Glut-4. A final hybridization with 18S ribosomal RNA (rRNA) cDNA was performed for normalization. Densitometric analysis of the autoradiograms corresponding to BAT from controls (filled bars) and BATIRKO mice (open bars) after normalization of arbitrary units with the amount of 18S rRNA detected is shown in lower panels. Results are expressed as mean ± SEM (n = 5–6). *P < 0.05; **P < 0.005; ***P < 0.001 BATIRKO vs control. Autoradiograms are representative of five experiments.
Figure 4
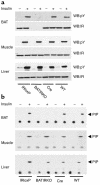
Insulin-stimulated tyrosine phosphorylation of the IR β-chain and PI 3-kinase activity. Six-month-old mice were anesthetized by intraperitoneal injection of pentobarbital and injected with either saline (–) or insulin (+) via the inferior vena cava. Protein extracts from liver, muscle, and BAT were subjected to immunoprecipitation with the monoclonal anti-IR Ab (a) or with a monoclonal anti-Tyr (P) Ab (b). The resulting immune complexes were separated by SDS-PAGE and analyzed by Western blot with the anti-Tyr (P) Ab or with the polyclonal anti-IR Ab (a), or washed and immediately used for an in vitro PI 3-kinase assay (b). This is representative of three experiments. WB, Western blot; pY, phospho-tyrosine; PIP, phosphatidylinositol phosphate.
Figure 5
BATIRKO mice demonstrate a progressive glucose intolerance. The ability to handle a glucose load was assessed by carrying out a glucose tolerance test at 3, 6, and 12 months of age in WT (×), Cre (triangles), IR_lox_P (diamonds), and BATIRKO (squares) male (a, c, and e) and female (b, d, and f) mice. An age-dependent glucose intolerance was observed in both male and female mice. Results are expressed as mean ± SEM (n = 10–20). ***P < 0.0005; #P < 0.001; **P < 0.005; *P < 0.05; BATIRKO vs. WT or IR_lox_P or Cre.
Figure 6
BATIRKO male mice show an insulin-secretion defect. A representative insulin-secretion test is shown. Results are expressed as mean ± SEM (n = 7–12). *P < 0.001; #P < 0.005 (a). Blood glucose level (b)(upper panel) and blood insulin level (b) (lower panel) were determined in fasted and fed control and BATIRKO mice. Results are expressed as mean ± SEM (n = 25–30), *P < 0.000001, #P < 0.01 BATIRKO vs. control. Islet morphology was studied by immunohistochemical analysis in BATIRKO and control mice, as shown in Methods. Representative pancreatic sections from control and diabetic and nondiabetic 6-month-old BATIRKO mice stained for insulin are shown (c)(upper panels). β-cell area was measured by immunohistochemical analysis. Results are expressed as the percentage of the total surveyed area containing cells positive for insulin and are mean ± SEM (n = 10). *P < 0.01. Both diabetic and nondiabetic animals were studied (c)(lower panel). Insulin secretion in control and BATIRKO isolated islets is shown. Results are expressed as mean ± SEM (n = 4). *P < 0.05 (d). RT-PCR analysis of total RNA from BAT of BATIRKO mice (BB) and islets from control (CI) and BATIRKO mice (BI) was performed, and IR expression was studied (e)(left panel). Total islet RNA from control (C) and BATIRKO (B) mice was submitted to Northern blot analysis and hybridized with labeled insulin and UCP-2 cDNAs. A representative experiment out of three is shown. Densitometric analysis was performed using 18S ribosomal rRNA for normalization (e)(right panels).
Similar articles
- Disruption of insulin signaling in Myf5-expressing progenitors leads to marked paucity of brown fat but normal muscle development.
Lynes MD, Schulz TJ, Pan AJ, Tseng YH. Lynes MD, et al. Endocrinology. 2015 May;156(5):1637-47. doi: 10.1210/en.2014-1773. Epub 2015 Jan 27. Endocrinology. 2015. PMID: 25625589 Free PMC article. - Essential Role of IGFIR in the Onset of Male Brown Fat Thermogenic Function: Regulation of Glucose Homeostasis by Differential Organ-Specific Insulin Sensitivity.
Viana-Huete V, Guillén C, García-Aguilar A, García G, Fernández S, Kahn CR, Benito M. Viana-Huete V, et al. Endocrinology. 2016 Apr;157(4):1495-511. doi: 10.1210/en.2015-1623. Epub 2016 Feb 24. Endocrinology. 2016. PMID: 26910308 Free PMC article. - Adipocyte insulin receptor activity maintains adipose tissue mass and lifespan.
Friesen M, Hudak CS, Warren CR, Xia F, Cowan CA. Friesen M, et al. Biochem Biophys Res Commun. 2016 Aug 5;476(4):487-492. doi: 10.1016/j.bbrc.2016.05.151. Epub 2016 May 28. Biochem Biophys Res Commun. 2016. PMID: 27246738 - The brown adipose cell: a unique model for understanding the molecular mechanism of insulin resistance.
Valverde AM, Benito M. Valverde AM, et al. Mini Rev Med Chem. 2005 Mar;5(3):269-78. doi: 10.2174/1389557053175380. Mini Rev Med Chem. 2005. PMID: 15777261 Review. - Tissue-specificity of insulin action and resistance.
Benito M. Benito M. Arch Physiol Biochem. 2011 Jul;117(3):96-104. doi: 10.3109/13813455.2011.563748. Epub 2011 Apr 20. Arch Physiol Biochem. 2011. PMID: 21506723 Review.
Cited by
- Disruption of insulin signaling in Myf5-expressing progenitors leads to marked paucity of brown fat but normal muscle development.
Lynes MD, Schulz TJ, Pan AJ, Tseng YH. Lynes MD, et al. Endocrinology. 2015 May;156(5):1637-47. doi: 10.1210/en.2014-1773. Epub 2015 Jan 27. Endocrinology. 2015. PMID: 25625589 Free PMC article. - Prolactin receptor signaling is essential for perinatal brown adipocyte function: a role for insulin-like growth factor-2.
Viengchareun S, Servel N, Fève B, Freemark M, Lombès M, Binart N. Viengchareun S, et al. PLoS One. 2008 Feb 6;3(2):e1535. doi: 10.1371/journal.pone.0001535. PLoS One. 2008. PMID: 18253483 Free PMC article. - Adrenoceptor regulation of the mechanistic target of rapamycin in muscle and adipose tissue.
Chia LY, Evans BA, Mukaida S, Bengtsson T, Hutchinson DS, Sato M. Chia LY, et al. Br J Pharmacol. 2019 Jul;176(14):2433-2448. doi: 10.1111/bph.14616. Epub 2019 Apr 7. Br J Pharmacol. 2019. PMID: 30740664 Free PMC article. Review. - Deletion of intestinal epithelial insulin receptor attenuates high-fat diet-induced elevations in cholesterol and stem, enteroendocrine, and Paneth cell mRNAs.
Andres SF, Santoro MA, Mah AT, Keku JA, Bortvedt AE, Blue RE, Lund PK. Andres SF, et al. Am J Physiol Gastrointest Liver Physiol. 2015 Jan 15;308(2):G100-11. doi: 10.1152/ajpgi.00287.2014. Epub 2014 Nov 13. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25394660 Free PMC article. - Essential Role of IGFIR in the Onset of Male Brown Fat Thermogenic Function: Regulation of Glucose Homeostasis by Differential Organ-Specific Insulin Sensitivity.
Viana-Huete V, Guillén C, García-Aguilar A, García G, Fernández S, Kahn CR, Benito M. Viana-Huete V, et al. Endocrinology. 2016 Apr;157(4):1495-511. doi: 10.1210/en.2015-1623. Epub 2016 Feb 24. Endocrinology. 2016. PMID: 26910308 Free PMC article.
References
- Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990;113:909–915. - PubMed
- Martin BC, Warram JH, Krolewski AS, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type II diabetes mellitus: results of 25 year follow-up study. Lancet. 1992;340:925–929. - PubMed
- Kahn CR. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43:1066–1084. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials