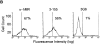Mannose receptor is a novel ligand for L-selectin and mediates lymphocyte binding to lymphatic endothelium - PubMed (original) (raw)
Mannose receptor is a novel ligand for L-selectin and mediates lymphocyte binding to lymphatic endothelium
H Irjala et al. J Exp Med. 2001.
Abstract
Continuous lymphocyte recirculation between blood and lymphoid tissues forms a basis for the function of the immune system. Lymphocyte entrance from the blood into the tissues has been thoroughly characterized, but mechanisms controlling lymphocyte exit from the lymphoid tissues via efferent lymphatics have remained virtually unknown. In this work we have identified mannose receptor (MR) on human lymphatic endothelium and demonstrate its involvement in binding of lymphocytes to lymphatic vessels. We also show that the binding requires L-selectin, and L-selectin and MR form a receptor-ligand pair. On the other hand, L-selectin binds to peripheral lymph node addressins (PNAds) on high endothelial venules (HEVs) that are sites where lymphocytes enter the lymphatic organs. Interestingly, MR is absent from HEVs and PNAds from lymphatic endothelium. Thus, lymphocyte L-selectin uses distinct ligand molecules to mediate binding at sites of lymphocyte entrance and exit within lymph nodes. Taken together, interaction between L-selectin and MR is the first molecularly defined mechanism mediating lymphocyte binding to lymphatic endothelium.
Figures
Figure 1
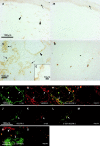
The monoclonal antibody 3-155 recognizes lymphatic endothelium both in afferent and efferent lymphatic systems. In indirect immunoperoxidase staining the afferent lymphatic sacs (arrowheads) in the skin stain positively (epidermis pointed out by an arrow). Some of the positive structures are cells belonging to macrophage lineage. (A) The staining with a negative control antibody does not show any specific reactivity (B). 3-155 reacts with small lymphatic vessels (cross-sectional profile, arrows), and with larger sinusoids (a single endothelial cell layer is seen, arrowhead) in the lymph node (C). The same area lacks any reactivity when stained with a negative control antibody (D). (E) The inset demonstrates expression of 3-155 antigen in sinusoidal endothelium with a high power magnification. In other organs studied sinusoidal endothelium of liver, occasional tissue macrophages, and lymphatic vessels stained positively with 3-155 (data not shown). Double stainings of lymphatic vessels in a lymph node (F–I) and skin (J–M) with 3-155, anti–VEGFR-3, and negative control antibodies (I and M). Two-color staining of lymph node sections with 3-155 (green), MECA-79 (against PNAd; red) (N) and negative control antibodies (O). The opposite phenotypes of lymphatic endothelium and HEVs regarding expression of MR and PNAds are obvious. Arrows point to the lymphatic endothelium (F, G, H, J, K, L, and N) and an arrowhead to HEVs (N).
Figure 2
The molecule recognized by 3-155 is involved in lymphocyte binding to lymphatic endothelium. An adhesion assay was performed to measure lymphocyte binding to lymphatic endothelium. In this assay the lymphocytes (some pointed out by arrows) specifically bind to endothelium of smaller lymphatic vessels that appear as bunches (A) or to endothelial cells that are lining the larger sinusoids (B) (see staining in Fig. 1 C for comparison of these two types of lymphatic vessels). After incubating the sections with 3-155 antibody the number of bound cells is decreased. Two sinuses with markedly decreased number of lymphocytes are indicated by arrowheads (C). In D, the results of three independent inhibition experiments with 3-155 hybridoma supernatant are shown as mean percentage of maximal binding ± SEM. (E) Concentration dependent-inhibition of 3-155.
Figure 2
The molecule recognized by 3-155 is involved in lymphocyte binding to lymphatic endothelium. An adhesion assay was performed to measure lymphocyte binding to lymphatic endothelium. In this assay the lymphocytes (some pointed out by arrows) specifically bind to endothelium of smaller lymphatic vessels that appear as bunches (A) or to endothelial cells that are lining the larger sinusoids (B) (see staining in Fig. 1 C for comparison of these two types of lymphatic vessels). After incubating the sections with 3-155 antibody the number of bound cells is decreased. Two sinuses with markedly decreased number of lymphocytes are indicated by arrowheads (C). In D, the results of three independent inhibition experiments with 3-155 hybridoma supernatant are shown as mean percentage of maximal binding ± SEM. (E) Concentration dependent-inhibition of 3-155.
Figure 3
3-155 antigen is an MR and macrophage and lymphatic MR have identical cell distribution and indistinguishable glycosylation profiles. (A) 3-155 antibody coupled to Sepharose 4B beads via rabbit anti–mouse IgG as well as the positive control precipitation with the commercial anti-MR antibody (same as the detecting antibody) were able to remove MR from lymphocyte lysate when tested by immunoblotting using a commercial anti-MR antibody. After the negative control precipitation with beads coupled to an irrelevant antibody (3G6) the MR band is clearly visible. (B) Histograms from flow cytometric analyses demonstrate that GM-CSF–activated monocytes show positive and identical staining both with a known anti–human MR antibody (α-hMR) and 3-155. 3G6 is a negative control antibody. (C) After various glycosidase treatments of activated monocyte (macrophages) and lymph node lysates (lymphatic), molecules were separated by SDS-PAGE and analyzed by immunoblotting with 3-155 and a negative control antibody (3G6).
Figure 3
3-155 antigen is an MR and macrophage and lymphatic MR have identical cell distribution and indistinguishable glycosylation profiles. (A) 3-155 antibody coupled to Sepharose 4B beads via rabbit anti–mouse IgG as well as the positive control precipitation with the commercial anti-MR antibody (same as the detecting antibody) were able to remove MR from lymphocyte lysate when tested by immunoblotting using a commercial anti-MR antibody. After the negative control precipitation with beads coupled to an irrelevant antibody (3G6) the MR band is clearly visible. (B) Histograms from flow cytometric analyses demonstrate that GM-CSF–activated monocytes show positive and identical staining both with a known anti–human MR antibody (α-hMR) and 3-155. 3G6 is a negative control antibody. (C) After various glycosidase treatments of activated monocyte (macrophages) and lymph node lysates (lymphatic), molecules were separated by SDS-PAGE and analyzed by immunoblotting with 3-155 and a negative control antibody (3G6).
Figure 3
3-155 antigen is an MR and macrophage and lymphatic MR have identical cell distribution and indistinguishable glycosylation profiles. (A) 3-155 antibody coupled to Sepharose 4B beads via rabbit anti–mouse IgG as well as the positive control precipitation with the commercial anti-MR antibody (same as the detecting antibody) were able to remove MR from lymphocyte lysate when tested by immunoblotting using a commercial anti-MR antibody. After the negative control precipitation with beads coupled to an irrelevant antibody (3G6) the MR band is clearly visible. (B) Histograms from flow cytometric analyses demonstrate that GM-CSF–activated monocytes show positive and identical staining both with a known anti–human MR antibody (α-hMR) and 3-155. 3G6 is a negative control antibody. (C) After various glycosidase treatments of activated monocyte (macrophages) and lymph node lysates (lymphatic), molecules were separated by SDS-PAGE and analyzed by immunoblotting with 3-155 and a negative control antibody (3G6).
Figure 4
L-selectin is a counter-receptor of MR on lymphatic endothelium. (A) Frozen section adhesion assays were performed by inhibiting lymphocyte binding to sinusoidal endothelium with 3-155, Dreg-56 (anti–L-selectin), both antibodies together, or with a control antibody (anti–human HLA ABC). The results of four independent assays are shown (mean percentage of maximal binding ± SEM). (B) 3-155 significantly inhibits binding of L-selectin positive cells to lymphatic endothelium. In contrast, only minor inhibition is seen when the binding of L-selectin negative cells is tested. Results of three independent assays are presented. L-selectin-IgM chimera was incubated on frozen section of a human lymph node. It binds to sinusoidal endothelial cells (arrow, C) to HEVs (pointed out by arrows, D). (E) Negative control. The L-selectin-IgM chimera coupled beads and control beads were used to deplete lymph node lysate and the nonprecipitated molecules were analyzed using immunoblotting and the monoclonal antibodies indicated (F).
Figure 4
L-selectin is a counter-receptor of MR on lymphatic endothelium. (A) Frozen section adhesion assays were performed by inhibiting lymphocyte binding to sinusoidal endothelium with 3-155, Dreg-56 (anti–L-selectin), both antibodies together, or with a control antibody (anti–human HLA ABC). The results of four independent assays are shown (mean percentage of maximal binding ± SEM). (B) 3-155 significantly inhibits binding of L-selectin positive cells to lymphatic endothelium. In contrast, only minor inhibition is seen when the binding of L-selectin negative cells is tested. Results of three independent assays are presented. L-selectin-IgM chimera was incubated on frozen section of a human lymph node. It binds to sinusoidal endothelial cells (arrow, C) to HEVs (pointed out by arrows, D). (E) Negative control. The L-selectin-IgM chimera coupled beads and control beads were used to deplete lymph node lysate and the nonprecipitated molecules were analyzed using immunoblotting and the monoclonal antibodies indicated (F).
Similar articles
- CD44 binds to macrophage mannose receptor on lymphatic endothelium and supports lymphocyte migration via afferent lymphatics.
Salmi M, Karikoski M, Elima K, Rantakari P, Jalkanen S. Salmi M, et al. Circ Res. 2013 Jun 7;112(12):1577-82. doi: 10.1161/CIRCRESAHA.111.300476. Epub 2013 Apr 19. Circ Res. 2013. PMID: 23603511 - P-selectin glycoprotein ligand-1 mediates L-selectin-independent leukocyte rolling in high endothelial venules of peripheral lymph nodes.
Harakawa N, Shigeta A, Wato M, Merrill-Skoloff G, Furie BC, Furie B, Okazaki T, Domae N, Miyasaka M, Hirata T. Harakawa N, et al. Int Immunol. 2007 Mar;19(3):321-9. doi: 10.1093/intimm/dxl149. Epub 2007 Jan 30. Int Immunol. 2007. PMID: 17267415 - Vascular adhesion protein 1 (VAP-1) mediates lymphocyte subtype-specific, selectin-independent recognition of vascular endothelium in human lymph nodes.
Salmi M, Tohka S, Berg EL, Butcher EC, Jalkanen S. Salmi M, et al. J Exp Med. 1997 Aug 18;186(4):589-600. doi: 10.1084/jem.186.4.589. J Exp Med. 1997. PMID: 9254657 Free PMC article. - High endothelial venules (HEVs): specialized endothelium for lymphocyte migration.
Girard JP, Springer TA. Girard JP, et al. Immunol Today. 1995 Sep;16(9):449-57. doi: 10.1016/0167-5699(95)80023-9. Immunol Today. 1995. PMID: 7546210 Review. - Biosynthesis of sulfated L-selectin ligands in human high endothelial venules (HEV).
Girard JP, Amalric F. Girard JP, et al. Adv Exp Med Biol. 1998;435:55-62. doi: 10.1007/978-1-4615-5383-0_6. Adv Exp Med Biol. 1998. PMID: 9498065 Review.
Cited by
- Ginseng polysaccharides: Potential antitumor agents.
Tao R, Lu K, Zong G, Xia Y, Han H, Zhao Y, Wei Z, Lu Y. Tao R, et al. J Ginseng Res. 2023 Jan;47(1):9-22. doi: 10.1016/j.jgr.2022.07.002. Epub 2022 Jul 16. J Ginseng Res. 2023. PMID: 36644386 Free PMC article. Review. - Endothelial cells from cord blood CD133+CD34+ progenitors share phenotypic, functional and gene expression profile similarities with lymphatics.
Nguyen VA, Fürhapter C, Obexer P, Stössel H, Romani N, Sepp N. Nguyen VA, et al. J Cell Mol Med. 2009 Mar;13(3):522-34. doi: 10.1111/j.1582-4934.2008.00340.x. J Cell Mol Med. 2009. PMID: 18410526 Free PMC article. - IL-12 directs further maturation of ex vivo differentiated NK cells with improved therapeutic potential.
Lehmann D, Spanholtz J, Sturtzel C, Tordoir M, Schlechta B, Groenewegen D, Hofer E. Lehmann D, et al. PLoS One. 2014 Jan 31;9(1):e87131. doi: 10.1371/journal.pone.0087131. eCollection 2014. PLoS One. 2014. PMID: 24498025 Free PMC article. - Transcription factor induction of vascular blood stem cell niches in vivo.
Hagedorn EJ, Perlin JR, Freeman RJ, Wattrus SJ, Han T, Mao C, Kim JW, Fernández-Maestre I, Daily ML, D'Amato C, Fairchild MJ, Riquelme R, Li B, Ragoonanan DAVE, Enkhbayar K, Henault EL, Wang HG, Redfield SE, Collins SH, Lichtig A, Yang S, Zhou Y, Kunar B, Gomez-Salinero JM, Dinh TT, Pan J, Holler K, Feldman HA, Butcher EC, van Oudenaarden A, Rafii S, Junker JP, Zon LI. Hagedorn EJ, et al. Dev Cell. 2023 Jun 19;58(12):1037-1051.e4. doi: 10.1016/j.devcel.2023.04.007. Epub 2023 Apr 28. Dev Cell. 2023. PMID: 37119815 Free PMC article. - Protein kinase D-dependent CXCR4 down-regulation upon BCR triggering is linked to lymphadenopathy in chronic lymphocytic leukaemia.
Saint-Georges S, Quettier M, Bouyaba M, Le Coquil S, Laurienté V, Guittat L, Lévy V, Ajchenbaum-Cymbalista F, Varin-Blank N, Le Roy C, Ledoux D. Saint-Georges S, et al. Oncotarget. 2016 Jul 5;7(27):41031-41046. doi: 10.18632/oncotarget.9031. Oncotarget. 2016. PMID: 27127886 Free PMC article.
References
- Butcher E.C. Leukocyte-endothelial cell recognitionthree (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. - PubMed
- Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigrationthe multistep paradigm. Cell. 1994;76:301–314. - PubMed
- Salmi M., Jalkanen S. How do lymphocytes know where to gocurrent concepts and enigmas of lymphocyte homing. Adv. Immunol. 1997;64:139–218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources