Induction of cell death in human immunodeficiency virus-infected macrophages and resting memory CD4 T cells by TRAIL/Apo2l - PubMed (original) (raw)
Induction of cell death in human immunodeficiency virus-infected macrophages and resting memory CD4 T cells by TRAIL/Apo2l
J J Lum et al. J Virol. 2001 Nov.
Abstract
Because the persistence of human immunodeficiency virus (HIV) in cellular reservoirs presents an obstacle to viral eradication, we evaluated whether tumor necrosis factor-related apoptosis-inducing ligand (TRAIL/Apo2L) induces apoptosis in such reservoirs. Lymphocytes and monocyte-derived macrophages (MDM) from uninfected donors do not die following treatment with either leucine zipper human TRAIL (LZhuTRAIL) or agonistic anti-TRAIL receptor antibodies. By contrast, such treatment induces apoptosis of in vitro HIV-infected MDM as well as peripheral blood lymphocytes from HIV-infected patients, including CD4(+) CD45RO(+) HLA-DR(-) lymphocytes. In addition, LZhuTRAIL-treated cells produce less viral RNA and p24 antigen than untreated controls. Whereas untreated cultures produce large amounts of HIV RNA and p24 antigen, of seven treated CD4(+) CD45RO(+) HLA-DR(-) cell cultures, viral RNA production was undetectable in all, p24 antigen was undetectable in six, and proviral DNA was undetectable in four. These data demonstrate that TRAIL induces death of cells from HIV-infected patients, including cell types which harbor latent HIV reservoirs.
Figures
FIG. 1
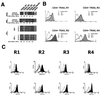
(A) Analysis of TRAIL-R1, TRAIL-R2, TRAIL-R3, TRAIL-R4, and TRAIL mRNA expression by RT-PCR. (Top) HIV-infected Jurkat T cells show increased message for TRAIL-R2, TRAIL-R3, and TRAIL/Apo2L compared to mock-infected controls (normalized to β-actin). (Center) HIV-1Bal-infected MDM show increased message for all TRAIL receptors and TRAIL/Apo2L compared to mock-infected controls. (Bottom) PBL from four HIV-1-positive individuals show increased message for TRAIL-R2, -R3, and -R4 or TRAIL/Apo2L compared to PBL from four HIV-1-negative individuals. (B) Cell surface expression of TRAIL-R1, -R2, -R3, and -R4 in CD4+ T cells from HIV-infected patients and healthy controls. Open histograms with dotted lines, cells from HIV-infected patients stained with an isotype control MAb; shaded histograms, cells from HIV-1-infected patients stained with anti-TRAIL receptor MAbs; open histograms with solid lines, cells from healthy controls stained with anti-TRAIL receptor MAbs. (C) Jurkat T cells (top) or PBL (bottom) from HIV-negative patients were treated with gp120 or a control (BSA) as indicated and analyzed by flow cytometry for TRAIL receptor expression. Open histograms with dotted lines, isotype control staining; open histograms with solid lines, cells treated with BSA and stained with the indicated anti-TRAIL receptor antibody; shaded histograms, cells treated with gp120 and stained with the indicated anti-TRAIL receptor antibody.
FIG. 2
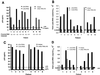
Sensitivity of PBL from HIV-1-infected donors to titrated doses of LZhuTRAIL. Cells were isolated from HIV-1-infected donors and incubated with increasing concentrations of LZhuTRAIL as indicated. Cell death was measured by Hoechst staining. Data are representative of three independent experiments. Spontaneous levels of apoptosis are indicated by asterisks.
FIG. 3
LZhuTRAIL induces apoptosis in cells from HIV-1-infected patients. PBL from 26 randomly selected HIV-1-infected patients or 5 uninfected controls were treated with 1 μg of LZhuTRAIL and analyzed for apoptosis by Hoechst staining, as were CD4+ T cells and CD8+ T cells.
FIG. 4
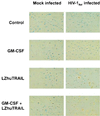
Induction of apoptosis in macrophages by LZhuTRAIL. MDM from 11 HIV-1-negative donors were mock or HIV-1Bal infected with or without GM-CSF. Fourteen days following infection, cells were treated with LZhuTRAIL and analyzed by TUNEL to determine the levels of apoptosis.
FIG. 5
LZhuTRAIL reduces viral gene expression in infected cells. CD4/DR− RO+ cells were isolated from seven HIV-1-infected patients, treated with LZhuTRAIL or agonistic TRAIL receptor antibodies (or isotype controls), and analyzed for p24 antigen production (A) and for the presence of integrated proviral DNA. (B) Viral RNA in culture supernatants. (C and D) Unfractionated cells from four HIV-1-infected patients with suppressed plasma viremia for more than 12 months were treated with or without LZhuTRAIL or agonistic MAbs to TRAIL-R2 (or isotype controls) and tested for p24 antigen (C) or viral RNA in culture supernatants (D).
Similar articles
- CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis.
Herbeuval JP, Grivel JC, Boasso A, Hardy AW, Chougnet C, Dolan MJ, Yagita H, Lifson JD, Shearer GM. Herbeuval JP, et al. Blood. 2005 Nov 15;106(10):3524-31. doi: 10.1182/blood-2005-03-1243. Epub 2005 Jul 26. Blood. 2005. PMID: 16046522 Free PMC article. - Are blockers of gp120/CD4 interaction effective inhibitors of HIV-1 immunopathogenesis?
Herbeuval JP, Shearer GM. Herbeuval JP, et al. AIDS Rev. 2006 Jan-Mar;8(1):3-8. AIDS Rev. 2006. PMID: 16736946 Review. - Critical contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to apoptosis of human CD4+ T cells in HIV-1-infected hu-PBL-NOD-SCID mice.
Miura Y, Misawa N, Maeda N, Inagaki Y, Tanaka Y, Ito M, Kayagaki N, Yamamoto N, Yagita H, Mizusawa H, Koyanagi Y. Miura Y, et al. J Exp Med. 2001 Mar 5;193(5):651-60. doi: 10.1084/jem.193.5.651. J Exp Med. 2001. PMID: 11238596 Free PMC article. - Colony-Stimulating Factor 1 Receptor Antagonists Sensitize Human Immunodeficiency Virus Type 1-Infected Macrophages to TRAIL-Mediated Killing.
Cunyat F, Rainho JN, West B, Swainson L, McCune JM, Stevenson M. Cunyat F, et al. J Virol. 2016 Jun 24;90(14):6255-6262. doi: 10.1128/JVI.00231-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27122585 Free PMC article. - TRAIL induces apoptosis and activation of NFkappaB.
Jeremias I, Debatin KM. Jeremias I, et al. Eur Cytokine Netw. 1998 Dec;9(4):687-8. Eur Cytokine Netw. 1998. PMID: 9889416 Review.
Cited by
- Apoptosis and Phagocytosis as Antiviral Mechanisms.
Nainu F, Ophinni Y, Shiratsuchi A, Nakanishi Y. Nainu F, et al. Subcell Biochem. 2023;106:77-112. doi: 10.1007/978-3-031-40086-5_3. Subcell Biochem. 2023. PMID: 38159224 - Detecting Sources of Immune Activation and Viral Rebound in HIV Infection.
Wietgrefe SW, Duan L, Anderson J, Marqués G, Sanders M, Cummins NW, Badley AD, Dobrowolski C, Karn J, Pagliuzza A, Chomont N, Sannier G, Dubé M, Kaufmann DE, Zuck P, Wu G, Howell BJ, Reilly C, Herschhorn A, Schacker TW, Haase AT. Wietgrefe SW, et al. J Virol. 2022 Aug 10;96(15):e0088522. doi: 10.1128/jvi.00885-22. Epub 2022 Jul 20. J Virol. 2022. PMID: 35856674 Free PMC article. - Biomarkers of Activation and Inflammation to Track Disparity in Chronological and Physiological Age of People Living With HIV on Combination Antiretroviral Therapy.
Thurman M, Johnson S, Acharya A, Pallikkuth S, Mahesh M, Byrareddy SN. Thurman M, et al. Front Immunol. 2020 Oct 9;11:583934. doi: 10.3389/fimmu.2020.583934. eCollection 2020. Front Immunol. 2020. PMID: 33162998 Free PMC article. Review. - Systemic Inflammation and the Increased Risk of Inflamm-Aging and Age-Associated Diseases in People Living With HIV on Long Term Suppressive Antiretroviral Therapy.
Babu H, Ambikan AT, Gabriel EE, Svensson Akusjärvi S, Palaniappan AN, Sundaraj V, Mupanni NR, Sperk M, Cheedarla N, Sridhar R, Tripathy SP, Nowak P, Hanna LE, Neogi U. Babu H, et al. Front Immunol. 2019 Aug 27;10:1965. doi: 10.3389/fimmu.2019.01965. eCollection 2019. Front Immunol. 2019. PMID: 31507593 Free PMC article. - TRAILshort Protects against CD4 T Cell Death during Acute HIV Infection.
Natesampillai S, Paim AC, Cummins NW, Chandrasekar AP, Bren GD, Lewin SR, Kiem HP, Badley AD. Natesampillai S, et al. J Immunol. 2019 Aug 1;203(3):718-724. doi: 10.4049/jimmunol.1900271. Epub 2019 Jun 12. J Immunol. 2019. PMID: 31189571 Free PMC article.
References
- Ashkenazi A, Pai R C, Fong S, Leung S, Lawrence D A, Marsters S A, Blackie C, Chang L, McMurtrey A E, Hebert A, DeForge L, Koumenis I L, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall R H. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Investig. 1999;104:155–162. - PMC - PubMed
- Badley A D, Pilon A A, Landay A, Lynch D H. Mechanisms of HIV associated lymphocyte apoptosis. Blood. 2000;96:2951–2964. - PubMed
- Cavert W, Notermans D W, Staskus K, Wietgrefe S W, Zupancic M, Gebhard K, Henry K, Zhang Z-Q, Mills R, McDade H, Schuwirth C M, Goudsmit J, Danner S A, Haase A T. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–964. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials