Clathrin exchange during clathrin-mediated endocytosis - PubMed (original) (raw)
Clathrin exchange during clathrin-mediated endocytosis
X Wu et al. J Cell Biol. 2001.
Abstract
During clathrin-mediated endocytosis, clathrin-coated pits invaginate to form clathrin-coated vesicles (CVs). Since clathrin-coated pits are planar structures, whereas CVs are spherical, there must be a structural rearrangement of clathrin as invagination occurs. This could occur through simple addition of clathrin triskelions to the edges of growing clathrin-coated pits with very little exchange occurring between clathrin in the pits and free clathrin in the cytosol, or it could occur through large scale exchange of free and bound clathrin. In the present study, we investigated this question by studying clathrin exchange both in vitro and in vivo. We found that in vitro clathrin in CVs and clathrin baskets do not exchange with free clathrin even in the presence of Hsc70 and ATP where partial uncoating occurs. However, surprisingly FRAP studies on clathrin-coated pits labeled with green fluorescent protein-clathrin light chains in HeLa cells show that even when endocytosis is blocked by expression of a dynamin mutant or depletion of cholesterol from the membrane, replacement of photobleached clathrin in coated pits on the membrane occurs at almost the same rate and magnitude as when endocytosis is occurring. Furthermore, very little of this replacement is due to dissolution of old pits and reformation of new ones; rather, it is caused by a rapid ATP-dependent exchange of clathrin in the pits with free clathrin in the cytosol. On the other hand, consistent with the in vitro data both potassium depletion and hypertonic sucrose, which have been reported to transform clathrin-coated pits into clathrin cages just below the surface of the plasma membrane, not only block endocytosis but also block exchange of clathrin. Taken together, these data show that ATP-dependent exchange of free and bound clathrin is a fundamental property of clathrin-coated pits, but not clathrin baskets, and may be involved in a structural rearrangement of clathrin as clathrin-coated pits invaginate.
Figures
Figure 1.
Exchange of clathrin does not occur to a significant extent in vitro. (Lane 1) 0.5 μM [14C]clathrin was added to 1.0 μM CVs for 10 min at 25°C at pH 7.0 in buffer A. (Lane 2) Supernatant obtained from the uncoating of 0.5 μM mixed AP-[14C]clathrin baskets was added to 1.0 μM CVs at pH 7.0. (Lane 3) Supernatant from uncoating of 0.5 μM mixed AP-[14C]clathrin baskets was added to 1.0 μM partially uncoated baskets in buffer A at pH 6.5. (Lane 4) Supernatant from the uncoating of 0.5 μM mixed AP-[14C]clathrin baskets was added to 1.0 μM CVs, which were partially uncoated at pH 6.5. (Lane 5) [14C]clathrin was added to crude brain homogenate immediately before the initial homogenization. (Lane 6) [14C]clathrin was added to the crude brain homogenate immediately after homogenization. (Lane 7) [14C]clathrin was added to crude brain homogenate after the first low speed spin.
Figure 2.
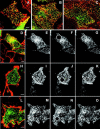
FRAP GFP-clathrin in HeLa cells at 37°C under different conditions. Control HeLa cells (A–C); HeLa expressing K44A- dynamin (D–F); and HeLa cells depleted of cholesterol (G–I). Images obtained directly before being photobleached (A, D, and G); images obtained immediately after photobleaching (B, E, and H); images obtained 4 min after photobleaching (C, F, and I). The photobleached area is indicated in each figure. Bars, 7 μm.
Figure 3.
Determination of the size of the clathrin-coated pits. CFP-clathrin in HeLa cells were imaged using the 413-nm laser as described in Materials and methods to maximize the resolution of the pit size. Images were acquired with a zoom such that each pixel repents 30 nm in length thereby enabling individual pits to be displayed clearly on the screen. HeLa cells (A); HeLa cells expressing WT dynamin (B); HeLa cells expressing K44A dynamin (C); HeLa cells depleted of cholesterol (D). Bar, 2 μm.
Figure 4.
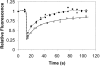
Kinetics of GFP-clathrin recovery after photobleaching at 37°C. HeLa cells (•), HeLa cells expressing WT dynamin (▴), HeLa cells expressing K44A-dynamin (▵), and HeLa cells depleted of cholesterol (○) were photobleached at 15 s and then scanned at low laser power.
Figure 5.
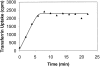
Single turnover of transferrin uptake at 28°C. 125I-labeled transferrin was preloaded onto HeLa cells at 4°C. The cells were then washed in PBS and transferred to 28°C. At the indicated times, the surface-bound transferrin was removed, cells were washed in PBS, and the internalized transferrin measured after solubilization of the cells.
Figure 6.
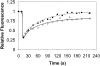
Kinetics of GFP-clathrin recovery after photobleaching at 28°C. HeLa cells (•), HeLa cells expressing K44A-dynamin (▵), and HeLa cells depleted of cholesterol (○) were photobleached at 15 s. After photobleaching, a time series of scanning were performed at low laser power.
Figure 7.
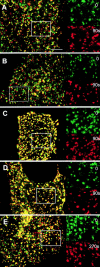
GFP-clathrin exchanges in cells in which transferrin uptake is blocked. Cy5-conjugated transferrin (4 μg/ml) was added to cholesterol-depleted HeLa cells (A and D–G), HeLa cells expressing K44A dynamin (B and H–K), or HeLa cells incubated and imaged at 15°C (C and L–O). Cholesterol-depleted cells or cells expressing K44A dynamin were incubated for 5 min at 37°C before being imaged at 28°C. (A–C) Basal surface of cells showing that transferrin is on the membrane and concentrates in most of the clathrin-coated pits; absence of transferrin internalization (D, H, and L); cells immediately before photobleaching (E, I, and M); cells immediately after photobleaching (F, J, and N); cells 5 min after photobleaching (G,K, and O). The photobleached area is indicated in each figure Bars: (A–C) 5 μm; (D–K) 6 μm; (L–O) 5 μm.
Figure 8.
CFP-clathrin–coated pit pattern is not changed significantly in cells undergoing exchange when endocytosis is blocked. Control HeLa (A); HeLa expressing WT dynamin (B); HeLa expressing K44A dynamin (C); cholesterol depleted HeLa (D); HeLa cells incubated at 15°C (E). Cells were imaged with a 63×,1.4 NA objective as described in Materials and methods, and images collected were overlayed at 0 and 90 s in A–D and 0 and 270 s in E. The inset magnifies the boxed area (length of box is 8 μm) to show the distribution of clathrin-coated pits at the two different times. Bar, 5 μm.
Figure 9.
Absence of clathrin exchange in cells treated with hypertonic sucrose, depleted of K + , or depleted of ATP. HeLa cells were treated as described in Materials and methods. Cells treated with hypertonic sucrose (A and C); cells depleted of K+ (D–F); cells depleted of ATP (G–I). Cells were imaged at 28°C before photobleaching (A, D, and G), immediately after photobleaching (B, E, and H), and 5 min after photobleaching (C,F,I). The photobleached area is indicated in each figure. Bars, 5 μm.
Figure 10.
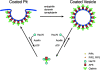
Model of clathrin exchange in vivo. See text for details.
Similar articles
- Exchange of clathrin, AP2 and epsin on clathrin-coated pits in permeabilized tissue culture cells.
Yim YI, Scarselletta S, Zang F, Wu X, Lee DW, Kang YS, Eisenberg E, Greene LE. Yim YI, et al. J Cell Sci. 2005 Jun 1;118(Pt 11):2405-13. doi: 10.1242/jcs.02356. J Cell Sci. 2005. PMID: 15923653 - Depletion of GAK/auxilin 2 inhibits receptor-mediated endocytosis and recruitment of both clathrin and clathrin adaptors.
Lee DW, Zhao X, Zhang F, Eisenberg E, Greene LE. Lee DW, et al. J Cell Sci. 2005 Sep 15;118(Pt 18):4311-21. doi: 10.1242/jcs.02548. J Cell Sci. 2005. PMID: 16155256 - Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits.
Merrifield CJ, Feldman ME, Wan L, Almers W. Merrifield CJ, et al. Nat Cell Biol. 2002 Sep;4(9):691-8. doi: 10.1038/ncb837. Nat Cell Biol. 2002. PMID: 12198492 - Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis.
Eisenberg E, Greene LE. Eisenberg E, et al. Traffic. 2007 Jun;8(6):640-6. doi: 10.1111/j.1600-0854.2007.00568.x. Epub 2007 May 4. Traffic. 2007. PMID: 17488288 Review. - Cargo recognition during clathrin-mediated endocytosis: a team effort.
Sorkin A. Sorkin A. Curr Opin Cell Biol. 2004 Aug;16(4):392-9. doi: 10.1016/j.ceb.2004.06.001. Curr Opin Cell Biol. 2004. PMID: 15261671 Review.
Cited by
- A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors.
Keyel PA, Mishra SK, Roth R, Heuser JE, Watkins SC, Traub LM. Keyel PA, et al. Mol Biol Cell. 2006 Oct;17(10):4300-17. doi: 10.1091/mbc.e06-05-0421. Epub 2006 Jul 26. Mol Biol Cell. 2006. PMID: 16870701 Free PMC article. - Clathrin-adaptor ratio and membrane tension regulate the flat-to-curved transition of the clathrin coat during endocytosis.
Bucher D, Frey F, Sochacki KA, Kummer S, Bergeest JP, Godinez WJ, Kräusslich HG, Rohr K, Taraska JW, Schwarz US, Boulant S. Bucher D, et al. Nat Commun. 2018 Mar 16;9(1):1109. doi: 10.1038/s41467-018-03533-0. Nat Commun. 2018. PMID: 29549258 Free PMC article. - Auxilin depletion causes self-assembly of clathrin into membraneless cages in vivo.
Hirst J, Sahlender DA, Li S, Lubben NB, Borner GH, Robinson MS. Hirst J, et al. Traffic. 2008 Aug;9(8):1354-71. doi: 10.1111/j.1600-0854.2008.00764.x. Epub 2008 May 17. Traffic. 2008. PMID: 18489706 Free PMC article. - DASC, a sensitive classifier for measuring discrete early stages in clathrin-mediated endocytosis.
Wang X, Chen Z, Mettlen M, Noh J, Schmid SL, Danuser G. Wang X, et al. Elife. 2020 Apr 30;9:e53686. doi: 10.7554/eLife.53686. Elife. 2020. PMID: 32352376 Free PMC article. - Rapid and efficient clathrin-mediated endocytosis revealed in genome-edited mammalian cells.
Doyon JB, Zeitler B, Cheng J, Cheng AT, Cherone JM, Santiago Y, Lee AH, Vo TD, Doyon Y, Miller JC, Paschon DE, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Drubin DG. Doyon JB, et al. Nat Cell Biol. 2011 Mar;13(3):331-7. doi: 10.1038/ncb2175. Epub 2011 Feb 6. Nat Cell Biol. 2011. PMID: 21297641 Free PMC article.
References
- Brodin, L., P. Low, and O. Shupliakov. 2000. Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Curr. Opin. Neurobiol. 10:312–320. - PubMed
- Cao, T.T., R.W. Mays, and M. von Zastrow. 1998. Regulated endocytosis of G-protein-coupled receptors by a biochemically and functionally distinct subpopulation of clathrin-coated pits. J. Biol. Chem. 273:24952–24602. - PubMed
- Cremona, O., G. Di Paolo, M.R. Wenk, A. Luthi, W.T. Kim, K. Takei, L. Daniell, Y. Nemoto, S.B. Shears, R.A. Flavell, et al. 1999. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 99:179–188. - PubMed
- Damke, H., M. Gossen, S. Freundlieb, H. Bujard, and S.L. Schmid. 1995. Tightly regulated and inducible expression of dominant interfering dynamin mutant in stably transformed HeLa cells. Methods Enzymol. 257:209–220. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous