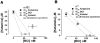Ketamine and its preservative, benzethonium chloride, both inhibit human recombinant alpha7 and alpha4beta2 neuronal nicotinic acetylcholine receptors in Xenopus oocytes - PubMed (original) (raw)
Ketamine and its preservative, benzethonium chloride, both inhibit human recombinant alpha7 and alpha4beta2 neuronal nicotinic acetylcholine receptors in Xenopus oocytes
K M Coates et al. Br J Pharmacol. 2001 Oct.
Abstract
1. Ketamine is a dissociative anaesthetic that is formulated as Ketalar, which contains the preservative benzethonium chloride (BCl). We have studied the effects of pure racemic ketamine, the preservative BCl and the Ketalar mixture on human neuronal nicotinic acetylcholine receptors (nAChRs) composed of the alpha7 subunit or alpha4 and beta2 subunits expressed in Xenopus laevis oocytes. 2. Ketamine inhibited responses to 1 mM acetylcholine (ACh) in both the human alpha7 and alpha4beta2 nAChRs, with IC(50) values of 20 and 50 microM respectively. Inhibition of the alpha7 nAChRs occurred within a clinically relevant concentration range, while inhibition of the alpha4beta2 nAChR was observed only at higher concentrations. The Ketalar formulation inhibited nAChR function more effectively than was expected given its ketamine concentration. The surprising increased inhibitory potency of Ketalar compared with pure ketamine appeared to be due to the activity of BCl, which inhibited both alpha7 (IC(50) value of 122 nM) and alpha4beta2 (IC(50) value of 49 nM) nAChRs at concentrations present in the clinical formulation of Ketalar. 3. Ketamine is a noncompetitive inhibitor at both the alpha7 and alpha4beta2 nAChR. In contrast, BCl causes a parallel shift in the ACh dose-response curve at the alpha7 nAChR suggesting competitive inhibition. Ketamine causes both voltage-dependent and use-dependent inhibition, only in the alpha4beta2 nAChR. 4. Since alpha7 nAChRs are likely to be inhibited during clinical use of Ketalar, the actions of ketamine and BCl on this receptor subtype may play a role in the profound analgesia, amnesia, immobility and/or autonomic modulation produced by this anaesthetic.
Figures
Figure 1
Chemical structures of acetylcholine, the native nicotinic agonist and benzethonium chloride, a nicotinic and muscarinic antagonist.
Figure 2
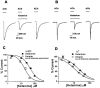
Ketalar, the clinically used formulation of ketamine, produced more potent inhibition of the activation of both the α4β2 and α7 nAChRs than ketamine. (A) A control current activated by 1 m
M
ACh, from α4β2 before (left), during a 2 s ACh application with 50 μ
M
ketamine (middle), and after ketamine washout (right). (B) A control current activated by 1 m
M
ACh, from α7 before (left), during a 2 s application of 20 μ
M
ketamine (middle), and after ketamine washout (right). (C) Concentration-response relationship for ketamine and Ketalar inhibition of the activation of the α4β2 nAChR, the IC50 for Ketalar was 17±3 μ
M
(Hill coefficient is 1.59±0.48) compared to an IC50 of 50±4 μ
M
(Hill coefficient is 1.55±0.22) for ketamine. (D) For the α7 nAChR, the IC50 for Ketalar was 11±0.6 μ
M
(Hill coefficient is 1.02±0.06) compared to an IC50 of 20±2 μ
M
(Hill coefficient is 1.07±0.14) for ketamine (number of oocytes for each data point (n)=5 – 7). Values are mean±s.e.
Figure 3
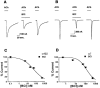
Benzethonium chloride (BCl), the preservative in Ketalar, caused inhibition of the activation of both the α7 and α4β2 nAChRs at concentrations consistent with those found in the clinically used Ketalar formulation. (A) A control current activated by 1 m
M
ACh, from α4β2 before (left), during a 2 s ACh application with 55 n
M
BCl (middle), and after BCl washout (right). (B) A control current activated by 1 m
M
ACh, from α7 before (left), during a 2 s ACh application with 110 n
M
BCl (middle), and after BCl washout (right). (C) A concentration-response curve for BCl inhibition of the activation of the α4β2 nAChR. For the α4β2 nAChR, the IC50 for BCl inhibition with 1 m
M
ACh was 49±11 n
M
(Hill coefficient is 0.67±0.11). (D) For the α7 nAChR, the IC50 for BCl inhibition with 1 m
M
ACh was 122±19 n
M
(Hill coefficient is 1.07±0.20) (_n_=5 – 7). Values are mean±s.e.
Figure 4
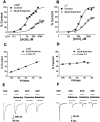
Ketamine inhibits ACh responses of the α4β2 and α7 nAChRs in a noncompetitive manner and this inhibition is voltage- and use-dependent in the α4β2 subtype. ACh dose-response curves were normalized to the average response to a saturating concentration of ACh in the absence of ketamine. (A) A concentration-response curve for the activation of α4β2 by varying concentrations of ACh in the absence and presence of 60 μ
M
ketamine. Membrane potential was held at −60 mV (_n_=5 – 6). (B) A concentration-response curve for the activation of α7 by varying ACh concentrations with and without 25 μ
M
ketamine. Membrane potential was held at −60 mV (_n_=5 – 6). (C) Voltage response relationship for the α4β2 nAChR inhibition by 60 μ
M
ketamine when activated by 1 m
M
ACh. At more negative membrane potentials, inhibition is more pronounced (P<0.0001) (_n_=4 – 7). (D) Voltage response relationship for the α7 nAChR inhibition by 20 μ
M
ketamine when activated by 1 m
M
ACh. There is no significant voltage-dependence as the slope is not significantly different from zero (_P_>0.05) (_n_=4 – 7). (E) Current traces of α4β2 in response to 1 m
M
ACh before the ketamine application (first), during the first 50 μ
M
ketamine application (second), during the second and third applications of continuous ketamine exposure (third and fourth figures). (F) Current traces of the α7 subtype in response to 1 m
M
ACh before the ketamine application (first), during the first 50 μ
M
ketamine application (second), during the second and third applications of continuous ketamine exposure (third and fourth figures). Values are mean±s.e.
Figure 5
Benzethonium chloride inhibits acetylcholine responses in the α7 nAChRs in a mixed competitive and non-competitive manner but there is no voltage- or use-dependence of the response in either subtype. ACh dose-response curves were normalized to the average response to a saturating concentration of ACh in the absence of ketamine. (A) A concentration-response curve for the activation of the α4β2 nAChR with varying concentrations of ACh in the absence and presence of 55 n
M
BCl. Membrane potential was held at −60 mV (_n_=5 – 7). (B) A concentration-response curve for the activation of the α7 nAChR by varying ACh concentrations with and without 110 n
M
BCl. Membrane potential was held at −60 mV (_n_=5 – 7). (C) Voltage response relationship for the α4β2 nAChR inhibition by 55 n
M
BCl when activated by 1 m
M
ACh. There is no significant voltage-dependence (_P_>0.05) (_n_=5 – 7). (D) Voltage response relationship for the α7 nAChR inhibition by 55 n
M
BCl when activated by 1 m
M
ACh. There is no significant voltage-dependence (_P_>0.05) (_n_=5 – 7). (E) Current traces from the α4β2 nAChR in response to 1 m
M
ACh before BCl application (first), during the first 5 n
M
BCl application (second), during the second and third applications of continuous BCl exposure (third and fourth figures). (F) Currents traces from the α7 nAChR in response to 1 m
M
ACh before the BCl application (first), during the first 100 n
M
BCl application (second), during the second and third applications of continuous BCl exposure (third and fourth figures). Values are mean±s.e.
Figure 6
A graphical analysis of ketamine and benzethonium chloride's interactions as seen in the α4β2 and α7 nAChRs. (A) Using 1 m
M
ACh as the agonist, the IC50 concentrations of ketamine alone, BCl alone and the combined drugs for inhibition of the α4β2 nAChR are plotted. The 95% confidence intervals for additivity are displayed as dotted lines. A subadditive effect between ketamine and BCl for the α4β2 subtype is shown. (B) Using 1 m
M
ACh as the agonist, the IC50 concentrations of ketamine alone, BCl alone and the combined drugs for inhibition of the α7 nAChR are plotted. The 95% confidence intervals for additivity are displayed as dotted lines. The IC50 concentrations for the combined drugs fall within the 95% confidence intervals, suggesting additivity. Error bars represent standard error.
Similar articles
- Synergistic inhibition of muscarinic signaling by ketamine stereoisomers and the preservative benzethonium chloride.
Durieux ME, Nietgen GW. Durieux ME, et al. Anesthesiology. 1997 Jun;86(6):1326-33. doi: 10.1097/00000542-199706000-00014. Anesthesiology. 1997. PMID: 9197302 - Untranslated region-dependent exclusive expression of high-sensitivity subforms of alpha4beta2 and alpha3beta2 nicotinic acetylcholine receptors.
Briggs CA, Gubbins EJ, Marks MJ, Putman CB, Thimmapaya R, Meyer MD, Surowy CS. Briggs CA, et al. Mol Pharmacol. 2006 Jul;70(1):227-40. doi: 10.1124/mol.105.020198. Epub 2006 Mar 28. Mol Pharmacol. 2006. PMID: 16569710 - Rat nicotinic acetylcholine receptor alpha2beta2 channels: comparison of functional properties with alpha4beta2 channels in Xenopus oocytes.
Khiroug SS, Khiroug L, Yakel JL. Khiroug SS, et al. Neuroscience. 2004;124(4):817-22. doi: 10.1016/j.neuroscience.2004.01.017. Neuroscience. 2004. PMID: 15026122 - The role of nicotinic acetylcholine receptors in the mechanisms of anesthesia.
Tassonyi E, Charpantier E, Muller D, Dumont L, Bertrand D. Tassonyi E, et al. Brain Res Bull. 2002 Jan 15;57(2):133-50. doi: 10.1016/s0361-9230(01)00740-7. Brain Res Bull. 2002. PMID: 11849819 Review. - CNS localization of neuronal nicotinic receptors.
Nashmi R, Lester HA. Nashmi R, et al. J Mol Neurosci. 2006;30(1-2):181-4. doi: 10.1385/JMN:30:1:181. J Mol Neurosci. 2006. PMID: 17192671 Review.
Cited by
- Ketamine Inhibition of the Pentameric Ligand-Gated Ion Channel GLIC.
Ion BF, Wells MM, Chen Q, Xu Y, Tang P. Ion BF, et al. Biophys J. 2017 Aug 8;113(3):605-612. doi: 10.1016/j.bpj.2017.06.041. Biophys J. 2017. PMID: 28793215 Free PMC article. - Ketamine: The final frontier or another depressing end?
Sial OK, Parise EM, Parise LF, Gnecco T, Bolaños-Guzmán CA. Sial OK, et al. Behav Brain Res. 2020 Apr 6;383:112508. doi: 10.1016/j.bbr.2020.112508. Epub 2020 Feb 1. Behav Brain Res. 2020. PMID: 32017978 Free PMC article. Review. - Taurine, an essential β-amino acid insulates against ketamine-induced experimental psychosis by enhancement of cholinergic neurotransmission, inhibition of oxidative/nitrergic imbalances, and suppression of COX-2/iNOS immunoreactions in mice.
Ben-Azu B, Adebayo OG, Jarikre TA, Oyovwi MO, Edje KE, Omogbiya IA, Eduviere AT, Moke EG, Chijioke BS, Odili OS, Omondiabge OP, Oyovbaire A, Esuku DT, Ozah EO, Japhet K. Ben-Azu B, et al. Metab Brain Dis. 2022 Dec;37(8):2807-2826. doi: 10.1007/s11011-022-01075-5. Epub 2022 Sep 3. Metab Brain Dis. 2022. PMID: 36057735 - Ketamine preservative benzethonium chloride potentiates hippocampal synaptic transmission and binds neurotransmitter receptors and transporters.
Brown KA, Zanos P, Powels CF, Fix CJ, Michaelides M, Pereira EFR, Moaddel R, Gould TD. Brown KA, et al. Neuropharmacology. 2023 Mar 1;225:109403. doi: 10.1016/j.neuropharm.2022.109403. Epub 2022 Dec 21. Neuropharmacology. 2023. PMID: 36565852 Free PMC article. Review. - Modeling neuronal nicotinic and GABA receptors: important interface salt-links and protein dynamics.
Law RJ, Lightstone FC. Law RJ, et al. Biophys J. 2009 Sep 16;97(6):1586-94. doi: 10.1016/j.bpj.2009.06.044. Biophys J. 2009. PMID: 19751663 Free PMC article.
References
- BUDAVARI S.Benzethonium chloride Merck Index 12th edition 1996Whitehouse Station, NJ: Merck; ed. Budavari, S. pp1103
- DURIEUX M.E. Inhibition by ketamine of muscarinic acetylcholine receptor function. Anesth. Analg. 1995;81:57–62. - PubMed
- DURIEUX M.E., NIETGEN G.W. Synergistic inhibition of muscarinic signaling by ketamine stereoisomers and the preservative benzethonium chloride. Anesthesiology. 1997;86:1326–1333. - PubMed
- ELDER R.L. Safety assessment of cosmetic ingredients, final report on the safety assessment of benzethonium chloride and methylbenzethonium chloride. J. Am. Coll. Toxicol. 1985;4:65–106.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources