Helicobacter typhlonius sp. nov., a Novel Murine Urease-Negative Helicobacter Species - PubMed (original) (raw)
Helicobacter typhlonius sp. nov., a Novel Murine Urease-Negative Helicobacter Species
C L Franklin et al. J Clin Microbiol. 2001 Nov.
Abstract
Over the past decade, several Helicobacter species have been isolated from rodents. With the advent of PCR for the diagnosis of infectious agents, it has become clear that several previously uncharacterized Helicobacter species also colonize rodents. In this report, we describe a novel urease-negative helicobacter, Helicobacter typhlonius sp. nov., which was isolated from colonies of laboratory mice independently by two laboratories. Infection of immunodeficient mice by this bacterium resulted in typhlocolitis similar to that observed with other helicobacter infections. H. typhlonius is genetically most closely related to H. hepaticus. Like H. hepaticus, it is a spiral bacterium with bipolar sheathed flagella. However, this novel species contains a large intervening sequence in its 16S rRNA gene and is biochemically distinct from H. hepaticus. Notably, H. typhlonius does not produce urease or H(2)S nor does it hydrolize indoxyl-acetate. Compared to other Helicobacter species that commonly colonize rodents, H. typhlonius was found to be less prevalent than H. hepaticus and H. rodentium but as prevalent as H. bilis. H. typhlonius joins a growing list of helicobacters that colonize mice and are capable of inducing enteric disease in various strains of immunodeficient mice.
Figures
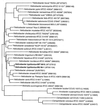
FIG. 1
Phylogenetic tree of members of the genera Helicobacter, Campylobacter, “Flexispira,” Wolinella, and Arcobacter based on 16S ribosomal DNA sequences and prepared by using the neighbor-joining method. Phylogenetic distances between bacteria are calculated by totaling horizontal branches between bacteria. Bar, 1 nucleotide substitution per 100 nucleotides.
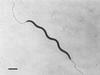
FIG. 2
Negatively stained preparation of H. typhlonius MU 96-1 demonstrating single sheathed bipolar flagella and no periplasmic fibers. Bar, 1 μm.
Similar articles
- Helicobacter ganmani sp. nov., a urease-negative anaerobe isolated from the intestines of laboratory mice.
Robertson BR, O'Rourke JL, Vandamme P, On SL, Lee A. Robertson BR, et al. Int J Syst Evol Microbiol. 2001 Sep;51(Pt 5):1881-1889. doi: 10.1099/00207713-51-5-1881. Int J Syst Evol Microbiol. 2001. PMID: 11594622 - Enteric lesions in SCID mice infected with "Helicobacter typhlonicus," a novel urease-negative Helicobacter species.
Franklin CL, Riley LK, Livingston RS, Beckwith CS, Hook RR Jr, Besch-Williford CL, Hunziker R, Gorelick PL. Franklin CL, et al. Lab Anim Sci. 1999 Oct;49(5):496-505. Lab Anim Sci. 1999. PMID: 10551450 - A novel enterohepatic Helicobacter species 'Helicobacter mastomyrinus' isolated from the liver and intestine of rodents.
Shen Z, Xu S, Dewhirst FE, Paster BJ, Pena JA, Modlin IM, Kidd M, Fox JG. Shen Z, et al. Helicobacter. 2005 Feb;10(1):59-70. doi: 10.1111/j.1523-5378.2005.00292.x. Helicobacter. 2005. PMID: 15691316 - Pathology, diagnosis and epidemiology of the rodent Helicobacter infection.
Zenner L. Zenner L. Comp Immunol Microbiol Infect Dis. 1999 Jan;22(1):41-61. doi: 10.1016/s0147-9571(98)00018-6. Comp Immunol Microbiol Infect Dis. 1999. PMID: 10099028 Review. - Gastric and enterohepatic non-Helicobacter pylori Helicobacters.
Flahou B, Haesebrouck F, Smet A, Yonezawa H, Osaki T, Kamiya S. Flahou B, et al. Helicobacter. 2013 Sep;18 Suppl 1:66-72. doi: 10.1111/hel.12072. Helicobacter. 2013. PMID: 24011248 Review.
Cited by
- Induction of colitis by a CD4+ T cell clone specific for a bacterial epitope.
Kullberg MC, Andersen JF, Gorelick PL, Caspar P, Suerbaum S, Fox JG, Cheever AW, Jankovic D, Sher A. Kullberg MC, et al. Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15830-5. doi: 10.1073/pnas.2534546100. Epub 2003 Dec 12. Proc Natl Acad Sci U S A. 2003. PMID: 14673119 Free PMC article. - Lurking in the shadows: emerging rodent infectious diseases.
Besselsen DG, Franklin CL, Livingston RS, Riley LK. Besselsen DG, et al. ILAR J. 2008;49(3):277-90. doi: 10.1093/ilar.49.3.277. ILAR J. 2008. PMID: 18506061 Free PMC article. - Methylation-Independent Chemotaxis Systems Are the Norm for Gastric-Colonizing Helicobacter Species.
Liu X, Ottemann KM. Liu X, et al. J Bacteriol. 2022 Sep 20;204(9):e0023122. doi: 10.1128/jb.00231-22. Epub 2022 Aug 16. J Bacteriol. 2022. PMID: 35972258 Free PMC article. - Dysbiosis exacerbates colitis by promoting ubiquitination and accumulation of the innate immune adaptor STING in myeloid cells.
Shmuel-Galia L, Humphries F, Lei X, Ceglia S, Wilson R, Jiang Z, Ketelut-Carneiro N, Foley SE, Pechhold S, Houghton J, Muneeruddin K, Shaffer SA, McCormick BA, Reboldi A, Ward D, Marshak-Rothstein A, Fitzgerald KA. Shmuel-Galia L, et al. Immunity. 2021 Jun 8;54(6):1137-1153.e8. doi: 10.1016/j.immuni.2021.05.008. Epub 2021 May 28. Immunity. 2021. PMID: 34051146 Free PMC article.
References
- Cox R A, Kempsell K, Fairclough L, Colston M J. The 16S ribosomal RNA of Mycobacterium leprae contains a unique sequence which can be used for identification by the polymerase chain reaction. J Med Microbiol. 1991;35:284–290. - PubMed
- Dewhirst F E, Fox J G, On S L. Recommended minimal standards for describing new species of the genus Helicobacter. Int J Syst Evol Microbiol. 2000;50(Pt. 6):2231–2237. - PubMed
- Eaton K A, Dewhirst F E, Radin M J, Fox J G, Paster B J, Krakowka S, Morgan D R. Helicobacter acinonyx sp. nov., isolated from cheetahs with gastritis. Int J Syst Bacteriol. 1993;43:99–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous