Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state - PubMed (original) (raw)
Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state
D A Hume et al. BMC Immunol. 2001.
Abstract
Background: Macrophages sense microorganisms through activation of members of the Toll-like receptor family, which initiate signals linked to transcription of many inflammation associated genes. In this paper we examine whether the signal from Toll-like receptors [TLRs] is sustained for as long as the ligand is present, and whether responses to different TLR agonists are additive.
Results: RAW264 macrophage cells were doubly-transfected with reporter genes in which the IL-12p40, ELAM or IL-6 promoter controls firefly luciferase, and the human IL-1beta promoter drives renilla luciferase. The resultant stable lines provide robust assays of macrophage activation by TLR stimuli including LPS [TLR4], lipopeptide [TLR2], and bacterial DNA [TLR9], with each promoter demonstrating its own intrinsic characteristics. With each of the promoters, luciferase activity was induced over an 8 hr period, and thereafter reached a new steady state. Elevated expression required the continued presence of agonist. Sustained responses to different classes of agonist were perfectly additive. This pattern was confirmed by measuring inducible cytokine production in the same cells. While homodimerization of TLR4 mediates responses to LPS, TLR2 appears to require heterodimerization with another receptor such as TLR6. Transient expression of constitutively active forms of TLR4 or TLR2 plus TLR6 stimulated IL-12 promoter activity. The effect of LPS, a TLR4 agonist, was additive with that of TLR2/6 but not TLR4, whilst that of lipopeptide, a TLR2 agonist, was additive with TLR4 but not TLR2/6. Actions of bacterial DNA were additive with either TLR4 or TLR2/6.
Conclusions: These findings indicate that maximal activation by any one TLR pathway does not preclude further activation by another, suggesting that common downstream regulatory components are not limiting. Upon exposure to a TLR agonist, macrophages enter a state of sustained activation in which they continuously sense the presence of a microbial challenge.
Figures
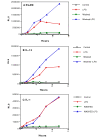
Figure 1
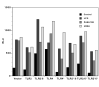
Time course of activation of integrated reporter genes in RAW264 cells. Pooled stable transfectants of RAW264 cells, with either the ELAM or IL-12 promoter driving firefly luciferase, cotransfected with the IL-1β promoter driving renilla luciferase, were cultured overnight as described in Materials and Methods. Where the cells were washed, the medium was aspirated, and replaced immediately with warm medium at time zero. A typical experiment of three is shown. Results for ELAM and IL12 promoters are the average of duplicates that differ by less than 10% from the mean. In the case of the IL-1β data, the results are the average of 4 datapoints obtained with the two separate pooled transfectant lines in the same experiment.
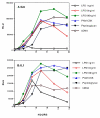
Figure 2
Time course of activation ofIL-6 and IL-1 promoters in RAW264 cells and the effect of a range of microbial agonists. Pooled stable transfectants with the IL-6 promoter driving firefly luciferase and the IL-6 promoter driving renilla luciferase were incubated overnight then stimulated with the agents shown for the time indicated prior to assay of luciferase activity. PMA3-CSK was added at 100 ng/ml, peptidoglycan at 10 μg/ml and E-coli genomic DNA (bDNA) at 10 μg/ml. A representative experiment of two is shown. Datapoints are the average of duplicate wells.
Figure 3
Comparative dose response curves for activation of IL-12, IL-1 and ELAM promoters. Pooled stable transfectants of RAW264 cells, with either the ELAM or IL-12 promoter driving firefly luciferase, cotransfected with the IL-1β promoter driving renilla luciferase, were cultured overnight as described in Materials and Methods. E. coli genomic DNA (bDNA), LPS or PAM3-CSK lipopeptide (PAM) were added at the concentrations indicated (LPS and PAM are in ng/ml, bDNA in μg/ml). After 8 hrs, the cells were harvested for determination of luciferase activity. Datapoints are the average of duplicate wells. The experiment is representative of two, and reiterates the pattern observed in 2 others with differences in design.
Figure 4
Additive effects of different microbial agonists and lack of evidence of tolerance in reporter gene activation. Pooled stable transfectants of RAW264 cells, with either the ELAM or IL-12 promoter driving firefly luciferase, cotransfected with the IL-1β promoter driving renilla luciferase, were cultured overnight for 16–18 hrs with either no addition, LPS (100 ng/ml), PAM3-CSK lipopeptide (PAM, 100 ng/ml) or E. coli genomic DNA (bDNA, 10 μg/ml) as indicated on the vertical legend. The cells were washed by aspirating the medium, replacing it with warm medium, incubating for 5 minutes and replacing the medium a second time. The replacement medium contained no additional stimulus, or one of original stimuli as indicated on the legend to the X-axis, so that a 4 × 4 matrix of pretreatment and retreatment was established. After a further 8 hrs stimulation, the cells were harvested for determination of luciferase activity. Each datapoint is the average of duplicate wells. The experiments is representative of two with identical design. The pattern of restimulation has been confirmed for LPS, and for the ELAM/IL-1 line in separate experiments (not shown).
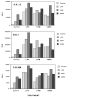
Figure 5
Combined effects of constitutively active toll-like receptors and added agonists. RAW264 cells were transiently co-transfected with the IL-12 promoter luciferase reporter genes and expression plasmids encoding wild-type and mutated (indicated by an asterisk) forms of the CD4/TLR chimaeric receptors as indicated either alone for TLR4 or in combination for TLR2 and 6. The experiment is described in detail in Materials and Methods. After overnight incubation, the cells were treated with either LPS [100 ng/ml], PAM3-CSK lipopeptide (100 ng/ml) or E-coli genomic DNA (10 μg/ml) as indicated. Each datapoint is the average of duplicate wells. The same pattern was observed in three separate experiments. The major variation between experiments was in the effect of the vector alone on basal reporter gene activity.
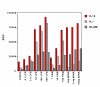
Figure 6
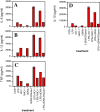
Combined effects of LPS and bacterial DNA on pro-inflammatory cytokine production Panels A-C. RAW264 cells were stimulated for 24 h with medium control, LPS [100 ng/ml], stimulatory CpG oligonucleotide AO-1 (3 μM), non-stimulatory GpC oligonucleotide NAO-1 (3 μM), E. coli DNA (bDNA, 5 μg/ml), DNase I-treated bDNA (5 μg/ml) or combinations as indicated. Cell supernatants were harvested and IL-6 (Panel A), IL-12 (Panel B] and TNF-β (Panel C) levels were estimated by ELISA. Data are mean of triplicates ± SD and similar results were obtained in 3 independent experiments. Panel D. Bone marrow-derived primary macrophages were stimulated with medium control, LPS (100 ng/ml), AO-1 (3 μM), NAO-1 (3 μM), E. coli DNA (bDNA, 5 μg/ml), DNase I-treated E. coli DNA (5 μg/ml), or combinations as indicated. After 24 h, cell supernatants were harvested and IL-12 levels in supernatants were estimated by ELISA. Data (mean of triplicates ± SD) are representative of 4 independent experiments.
Similar articles
- Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1.
Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Lee JY, et al. J Biol Chem. 2004 Apr 23;279(17):16971-9. doi: 10.1074/jbc.M312990200. Epub 2004 Feb 13. J Biol Chem. 2004. PMID: 14966134 - An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors.
Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ. Pinhal-Enfield G, et al. Am J Pathol. 2003 Aug;163(2):711-21. doi: 10.1016/S0002-9440(10)63698-X. Am J Pathol. 2003. PMID: 12875990 Free PMC article. - Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis.
Hajishengallis G, Sharma A, Russell MW, Genco RJ. Hajishengallis G, et al. Ann Periodontol. 2002 Dec;7(1):72-8. doi: 10.1902/annals.2002.7.1.72. Ann Periodontol. 2002. PMID: 16013219 Review. - Endogenous ligands of Toll-like receptors.
Tsan MF, Gao B. Tsan MF, et al. J Leukoc Biol. 2004 Sep;76(3):514-9. doi: 10.1189/jlb.0304127. Epub 2004 Jun 3. J Leukoc Biol. 2004. PMID: 15178705 Review.
Cited by
- Modeling the Bistable Dynamics of the Innate Immune System.
Kadelka S, Boribong BP, Li L, Ciupe SM. Kadelka S, et al. Bull Math Biol. 2019 Jan;81(1):256-276. doi: 10.1007/s11538-018-0527-y. Epub 2018 Nov 1. Bull Math Biol. 2019. PMID: 30387078 Free PMC article. - Plasmodium falciparum PfEMP1 Modulates Monocyte/Macrophage Transcription Factor Activation and Cytokine and Chemokine Responses.
Sampaio NG, Eriksson EM, Schofield L. Sampaio NG, et al. Infect Immun. 2017 Dec 19;86(1):e00447-17. doi: 10.1128/IAI.00447-17. Print 2018 Jan. Infect Immun. 2017. PMID: 29038124 Free PMC article. - Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury.
Vit JP, Ohara PT, Bhargava A, Kelley K, Jasmin L. Vit JP, et al. J Neurosci. 2008 Apr 16;28(16):4161-71. doi: 10.1523/JNEUROSCI.5053-07.2008. J Neurosci. 2008. PMID: 18417695 Free PMC article. - DNase Sda1 allows invasive M1T1 Group A Streptococcus to prevent TLR9-dependent recognition.
Uchiyama S, Andreoni F, Schuepbach RA, Nizet V, Zinkernagel AS. Uchiyama S, et al. PLoS Pathog. 2012;8(6):e1002736. doi: 10.1371/journal.ppat.1002736. Epub 2012 Jun 14. PLoS Pathog. 2012. PMID: 22719247 Free PMC article.
References
- Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol . 1996;60:8–26. - PubMed
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 [TLR4]-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. - PubMed
- Poltorak A, He X, Smimova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science . 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. - DOI - PubMed
- Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 [Tlr4] [see comments] [published erratum appears in J Exp Med 1999 May 3; 189 [9]: following 1518]. J Exp Med . 1999;189:615–625. doi: 10.1084/jem.189.4.615. - DOI - PMC - PubMed
- Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–3479. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases