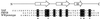Ultrafast folding of WW domains without structured aromatic clusters in the denatured state - PubMed (original) (raw)
Ultrafast folding of WW domains without structured aromatic clusters in the denatured state
N Ferguson et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Ultrafast-folding proteins are important for combining experiment and simulation to give complete descriptions of folding pathways. The WW domain family comprises small proteins with a three-stranded antiparallel beta-sheet topology. Previous studies on the 57-residue YAP 65 WW domain indicate the presence of residual structure in the chemically denatured state. Here we analyze three minimal core WW domains of 38-44 residues. There was little spectroscopic or thermodynamic evidence for residual structure in either their chemically or thermally denatured states. Folding and unfolding kinetics, studied by using rapid temperature-jump and continuous-flow techniques, show that each domain folds and unfolds very rapidly in a two-state transition through a highly compact transition state. Folding half-times were as short as 17 micros at 25 degrees C, within an order of magnitude of the predicted maximal rate of loop formation. The small size and topological simplicity of these domains, in conjunction with their very rapid two-state folding, may allow us to reduce the difference in time scale between experiment and theoretical simulation.
Figures
Figure 1
Sequence alignment of the YAP 65, FBP28, and prototype WW domains. Black boxes indicate identical residues. Asterisks mark the highly conserved tryptophan residues. In conjunction with other highly conserved residues (black circles), the tryptophan residues form the hydrophobic core (5). The positions of Strands I–III, connected by β-hairpins, are indicated above the sequence alignment.
Figure 2
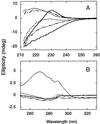
CD spectra of the YAP 65 WW domain under native and denaturing conditions. (A) Far-UV CD spectra of the YAP 65 WW domain at 298 K in 20 mM Mops, pH 7.0 (upper solid line); 371 K in 20 mM Mops, pH 7.0 (lower solid line); 298 K in 5.6 M GdmCl/20 mM Mops, pH 7.0 (- - - - -). Spectrum of solutions at 298 K containing free aromatic amino acids (_N_-acetyl tryptophanamide, _N-_acetyl tyrosinamide, and _N-_acetyl phenylalanine) at equimolar concentrations to the aromatic content of the YAP 65 WW domain, in the presence (— – — –) or absence (· · · ·) of 5.6 M GdmCl/20 mM Mops, pH 7.0. The difference spectrum (– · · · –) generated by subtracting the contribution of the free aromatic amino acids in 5.6 M GdmCl and at 298 K from that of the YAP 65 WW denatured state under identical conditions. Similar results were obtained for the wild-type FBP28 and prototype WW domains. (B) Near-UV spectra of the wild-type FBP28 WW domain under native and denaturing conditions at 298 K. The near-UV CD spectrum acquired in 20 mM Mops, pH 7.0 (· · · ·) and in 5.6 M GdmCl/20 mM Mops, pH 7.0 ( ). The near-UV CD spectrum of free aromatic amino acids at equimolar concentrations to the aromatic content of wild-type protein, in the presence (- - - - -) and absence of 5.6 M GdmCl (– · · · –), is similar to that of the chemically denatured domain. Similar results were obtained for the prototype WW domain.
). The near-UV CD spectrum of free aromatic amino acids at equimolar concentrations to the aromatic content of wild-type protein, in the presence (- - - - -) and absence of 5.6 M GdmCl (– · · · –), is similar to that of the chemically denatured domain. Similar results were obtained for the prototype WW domain.
Figure 3
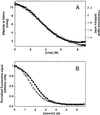
Thermal denaturation of the YAP 65 WW domain. Thick solid lines indicate the best fit of repeated thermal denaturation curves to a two-state transition assuming the experimentally determined Δ_C_ value (see Inset). The parameters from these fits were used to define the extrapolated baseline for the thermally denatured state (thin solid line). The denatured baseline coincides with the intrinsic ellipticity at 230 nm of the chemically denatured state (filled circle) after the intrinsic ellipticity of the aromatic acids has been subtracted (data from Fig. 2_A_ used). Similar results were observed for the other WW domains. (Inset) Dependence of Δ_H_D-N and T_m values (hollow circles) on pH for the wild-type FBP28 WW domain. Data were determined from thermal denaturation experiments monitored by using far-UV CD spectroscopy in the pH range 2.75–7.0. The gradient of the best line fit to the data (dashed line), weighted by the errors, defines the Δ_C
.
Figure 4
Urea-induced equilibrium denaturation of the prototype WW domain at 298 K. (A) Urea-induced denaturation of 10 μM protein in 20 mM sodium phosphate buffer, pH 7.0, was monitored by total fluorescence emission at >320 nm (filled circles) and far-UV CD spectroscopy at 230 nm (hollow circles). (B) GdmCl-induced equilibrium denaturation of wild-type (filled circles) and W30A mutant FBP28 WW domains (hollow circles) at 298 K. These data are normalized to allow plotting on a common scale. Solid lines indicate the best fit of each data set to a two-state transition. The Δ_G_D-N and _m_D-N values were within error irrespective of whether these data were fitted assuming nonsloping or sloping baselines (35) (Table 1).
Figure 5
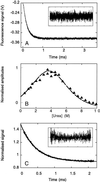
Relaxation kinetics for unfolding of the WW prototype domain in 2.0 M urea/20 mM sodium phosphate, pH 7.0. (A) Jumps (3°C) from 22 to 25°C were initiated by 30 kV discharges from a 20 nF capacitor. The average of eight traces is shown, omitting the initial fast change in signal that arises from the intrinsic temperature dependence of tryptophan fluorescence. The subsequent relaxation to the new equilibrium fits to a single exponential function (residuals, Inset) with k_obs = 5,210 ± 7 s−1. (B) The urea dependence of the kinetic unfolding amplitudes for the prototype WW domain. The kinetic amplitudes expected were simulated (broken line) by using only the equilibrium Δ_C, Δ_H_D-N, _T_m, and _m_D-N values and were highly consistent with the measured kinetic amplitudes (triangles). (C) Unfolding of the YAP 65 WW domain at 298 K measured by continuous-flow fluorescence spectroscopy. The fit of an individual unfolding trace (10 μM YAP 65 WW domain in 8.45 M urea, 20 mM phosphate buffer, pH 7.0) to a single exponential function is shown with _k_obs = 2,265 ± 8 s−1 (residuals, Inset).
Figure 6
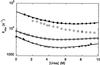
Dependence of log _k_obs on urea concentration for different WW domains at 298 K and pH 7.0. The data sets for the YAP 65 (triangles), prototype (squares), and W30A FBP28 (filled circles) WW domains were fitted to two-state transitions (as described in Materials and Methods). The high denaturation midpoint of the wild-type FBP28 WW domain (hollow circles) prevents the observed data from being accurately fitted. Filled triangles refer to YAP 65 WW kinetic data obtained by using continuous-flow measurements, hollow triangles to temperature-jump measurements.
Similar articles
- Understanding the mechanism of beta-hairpin folding via phi-value analysis.
Du D, Tucker MJ, Gai F. Du D, et al. Biochemistry. 2006 Feb 28;45(8):2668-78. doi: 10.1021/bi052039s. Biochemistry. 2006. PMID: 16489760 - Phi-analysis at the experimental limits: mechanism of beta-hairpin formation.
Petrovich M, Jonsson AL, Ferguson N, Daggett V, Fersht AR. Petrovich M, et al. J Mol Biol. 2006 Jul 21;360(4):865-81. doi: 10.1016/j.jmb.2006.05.050. Epub 2006 Jun 6. J Mol Biol. 2006. PMID: 16784750 - Folding kinetics of the SH3 domain of PI3 kinase by real-time NMR combined with optical spectroscopy.
Guijarro JI, Morton CJ, Plaxco KW, Campbell ID, Dobson CM. Guijarro JI, et al. J Mol Biol. 1998 Feb 27;276(3):657-67. doi: 10.1006/jmbi.1997.1553. J Mol Biol. 1998. PMID: 9551103 - Understanding the mechanism of beta-sheet folding from a chemical and biological perspective.
Jager M, Deechongkit S, Koepf EK, Nguyen H, Gao J, Powers ET, Gruebele M, Kelly JW. Jager M, et al. Biopolymers. 2008;90(6):751-8. doi: 10.1002/bip.21101. Biopolymers. 2008. PMID: 18844292 Review.
Cited by
- (Un)Folding mechanisms of the FBP28 WW domain in explicit solvent revealed by multiple rare event simulation methods.
Juraszek J, Bolhuis PG. Juraszek J, et al. Biophys J. 2010 Feb 17;98(4):646-56. doi: 10.1016/j.bpj.2009.10.039. Biophys J. 2010. PMID: 20159161 Free PMC article. - Non-natural amino acid fluorophores for one- and two-step fluorescence resonance energy transfer applications.
Rogers JM, Lippert LG, Gai F. Rogers JM, et al. Anal Biochem. 2010 Apr 15;399(2):182-9. doi: 10.1016/j.ab.2009.12.027. Epub 2009 Dec 28. Anal Biochem. 2010. PMID: 20036210 Free PMC article. - The role of the turn in beta-hairpin formation during WW domain folding.
Sharpe T, Jonsson AL, Rutherford TJ, Daggett V, Fersht AR. Sharpe T, et al. Protein Sci. 2007 Oct;16(10):2233-9. doi: 10.1110/ps.073004907. Epub 2007 Aug 31. Protein Sci. 2007. PMID: 17766370 Free PMC article. - Fast and faster: a designed variant of the B-domain of protein A folds in 3 microsec.
Arora P, Oas TG, Myers JK. Arora P, et al. Protein Sci. 2004 Apr;13(4):847-53. doi: 10.1110/ps.03541304. Protein Sci. 2004. PMID: 15044721 Free PMC article. - Protein folding kinetics: barrier effects in chemical and thermal denaturation experiments.
Naganathan AN, Doshi U, Muñoz V. Naganathan AN, et al. J Am Chem Soc. 2007 May 2;129(17):5673-82. doi: 10.1021/ja0689740. Epub 2007 Apr 10. J Am Chem Soc. 2007. PMID: 17419630 Free PMC article.
References
- Jackson S E. Folding Des. 1998;3:R81–R91. - PubMed
- Daggett V. Curr Opin Struct Biol. 2000;10:160–164. - PubMed
- Sudol M. Prog Biophys Mol Biol. 1996;65:113–132. - PubMed
- Macias M J, Hyvonen M, Baraldi E, Schultz J, Sudol M, Saraste M, Oschkinat H. Nature (London) 1996;382:646–649. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources