The crystal structure of human protein farnesyltransferase reveals the basis for inhibition by CaaX tetrapeptides and their mimetics - PubMed (original) (raw)
The crystal structure of human protein farnesyltransferase reveals the basis for inhibition by CaaX tetrapeptides and their mimetics
S B Long et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Protein farnesyltransferase (FTase) catalyzes the attachment of a farnesyl lipid group to the cysteine residue located in the C-terminal tetrapeptide of many essential signal transduction proteins, including members of the Ras superfamily. Farnesylation is essential both for normal functioning of these proteins, and for the transforming activity of oncogenic mutants. Consequently FTase is an important target for anti-cancer therapeutics. Several FTase inhibitors are currently undergoing clinical trials for cancer treatment. Here, we present the crystal structure of human FTase, as well as ternary complexes with the TKCVFM hexapeptide substrate, CVFM non-substrate tetrapeptide, and L-739,750 peptidomimetic with either farnesyl diphosphate (FPP), or a nonreactive analogue. These structures reveal the structural mechanism of FTase inhibition. Some CaaX tetrapeptide inhibitors are not farnesylated, and are more effective inhibitors than farnesylated CaaX tetrapeptides. CVFM and L-739,750 are not farnesylated, because these inhibitors bind in a conformation that is distinct from the TKCVFM hexapeptide substrate. This non-substrate binding mode is stabilized by an ion pair between the peptide N terminus and the alpha-phosphate of the FPP substrate. Conformational mapping calculations reveal the basis for the sequence specificity in the third position of the CaaX motif that determines whether a tetrapeptide is a substrate or non-substrate. The presence of beta-branched amino acids in this position prevents formation of the non-substrate conformation; all other aliphatic amino acids in this position are predicted to form the non-substrate conformation, provided their N terminus is available to bind to the FPP alpha-phosphate. These results may facilitate further development of FTase inhibitors.
Figures
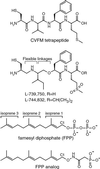
Figure 1
Chemical structures.
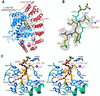
Figure 2
Structure of human FTase in complex with the CIFM-derived L-739,750 peptidomimetic and FPP substrate. (A) Overall structure. (B) 6σ FO-FC omit electron density for L-739,750. The FPP substrate and zinc ion are included for reference. (C) Residues forming van der Waals interactions with L-739,750, shown in stereo. The N terminus of the peptidomimetic forms an ion pair with an α-phosphate oxygen of the FPP substrate.
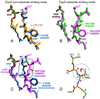
Figure 3
Comparison of CaaX non-substrate and substrate conformations. The superpositions are based on all FTase Cα atoms. The same orientation is shown in each panel. (A) CaaX tetrapeptide non-substrate inhibitor binding conformation. L-739,750 peptidomimetic and CVFM tetrapeptide shown with the FPP molecules observed in these two complexes colored yellow (with peptidomimetic) and blue (with CVFM tetrapeptide). Note that the valine to isoleucine substitution at the a1 position of the Ca1a2X motif does not affect the conformation of these two inhibitors, consistent with the orientation of these residues toward bulk solvent. (B) Peptide substrate binding conformation. TKCVFM and the K-Ras4B peptide TKCVIM (18) are both substrates and adopt the same backbone conformation. The N-terminal residue and the lysine side chain of each of these peptides are omitted from the figure for clarity. Also shown are the FPP analogs (Fig. 1), which are colored according to their observed conformations with these peptides. (C) Comparison of the hexapeptide substrate TKCVFM and the non-substrate tetrapeptide inhibitor CVFM. A boxed region highlights the differences between the substrate and non-substrate conformations. (D) Energetically unfavorable steric contact (red spikes) between the Cγ2 methyl group of the isoleucine residue in the a2 position and the carbonyl oxygen of the a1 residue when the phenylalanine residue of the CVFM tetrapeptide is replaced with isoleucine. Consequently, a tetrapeptide with a β-branched residue (isoleucine or valine) in the a2 position cannot adopt the non-substrate binding mode observed for CVFM. These steric contacts were displayed by using the programs
probe
and
reduce
(34) in conjunction with the program
o
(35).
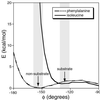
Figure 4
Conformational maps comparing placement of isoleucine (solid line) and phenylalanine (dashed line) in the a2 position of a tetrapeptide that adopts a non-substrate conformation. The coordinates of the CVFM peptide complex were used as a representative non-substrate conformation. Only the a1a2 dipeptide was considered in the calculation. Shown are the contributions to the van der Waals interactions as the φ dihedral angle between the a1 and a2 positions is varied. For each sampled value of this angle, the χ1 dihedral angles of the side chains in the a1 (valine) and a2 (isoleucine, valine, phenylalanine, leucine, or alanine) positions were allowed to vary within ±30° of the values observed in the crystal structure, and the minimum local energy was recorded. In the a2 position, valine resulted in a curve similar to isoleucine, whereas leucine and alanine yielded curves similar to phenylalanine (not shown). The shaded areas indicate the two regions in the vicinity of the non-substrate (φ = −154°, arrow) and substrate (φ = −126°, arrow) conformations, respectively (the conformational map outside these regions is not relevant, because the tetrapeptide is not observed to adopt such conformations in the FTase active site). Conformational maps were generated by using the
dezymer
program (36, 37).
Similar articles
- The basis for K-Ras4B binding specificity to protein farnesyltransferase revealed by 2 A resolution ternary complex structures.
Long SB, Casey PJ, Beese LS. Long SB, et al. Structure. 2000 Feb 15;8(2):209-22. doi: 10.1016/s0969-2126(00)00096-4. Structure. 2000. PMID: 10673434 - A non-peptide mimetic of Ras-CAAX: selective inhibition of farnesyltransferase and Ras processing.
Vogt A, Qian Y, Blaskovich MA, Fossum RD, Hamilton AD, Sebti SM. Vogt A, et al. J Biol Chem. 1995 Jan 13;270(2):660-4. doi: 10.1074/jbc.270.2.660. J Biol Chem. 1995. PMID: 7822292 - Advances in the development of farnesyltransferase inhibitors: substrate recognition by protein farnesyltransferase.
Yang W, Del Villar K, Urano J, Mitsuzawa H, Tamanoi F. Yang W, et al. J Cell Biochem Suppl. 1997;27:12-9. J Cell Biochem Suppl. 1997. PMID: 9591188 Review. - Structure based design of benzophenone-based non-thiol farnesyltransferase inhibitors.
Schlitzer M. Schlitzer M. Curr Pharm Des. 2002;8(19):1713-22. doi: 10.2174/1381612023394061. Curr Pharm Des. 2002. PMID: 12171543 Review.
Cited by
- Mediating kinase activity in Ras-mutant cancer: potential for an individualised approach?
Healy FM, Turner AL, Marensi V, MacEwan DJ. Healy FM, et al. Front Pharmacol. 2024 Sep 20;15:1441938. doi: 10.3389/fphar.2024.1441938. eCollection 2024. Front Pharmacol. 2024. PMID: 39372214 Free PMC article. Review. - Targeting Metalloenzymes for Therapeutic Intervention.
Chen AY, Adamek RN, Dick BL, Credille CV, Morrison CN, Cohen SM. Chen AY, et al. Chem Rev. 2019 Jan 23;119(2):1323-1455. doi: 10.1021/acs.chemrev.8b00201. Epub 2018 Sep 7. Chem Rev. 2019. PMID: 30192523 Free PMC article. Review. - Virtual screening and pharmacophore studies for ftase inhibitors using Indian plant anticancer compounds database.
Khan AH, Prakash A, Kumar D, Rawat AK, Srivastava R, Srivastava S. Khan AH, et al. Bioinformation. 2010 Jul 6;5(2):62-6. doi: 10.6026/97320630005062. Bioinformation. 2010. PMID: 21346865 Free PMC article. - Mutant HRas Signaling and Rationale for Use of Farnesyltransferase Inhibitors in Head and Neck Squamous Cell Carcinoma.
Wang J, Al-Majid D, Brenner JC, Smith JD. Wang J, et al. Target Oncol. 2023 Sep;18(5):643-655. doi: 10.1007/s11523-023-00993-3. Epub 2023 Sep 4. Target Oncol. 2023. PMID: 37665491 Review. - Convergent synthesis of aminomethylene peptidomimetics.
Assem N, Yudin AK. Assem N, et al. Nat Protoc. 2012 Jun 14;7(7):1327-34. doi: 10.1038/nprot.2012.066. Nat Protoc. 2012. PMID: 22722368
References
- Casey P J. Science. 1995;268:221–225. - PubMed
- Zhang F L, Casey P J. Annu Rev Biochem. 1996;65:241–269. - PubMed
- Ashar H R, James L, Gray K, Carr D, Black S, Armstrong L, Bishop W R, Kirschmeier P. J Biol Chem. 2000;275:30451–30457. - PubMed
- Schafer W R, Kim R, Sterne R, Thorner J, Kim S-H, Rine J. Science. 1989;245:379–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases