Crystal structure of a dynamin GTPase domain in both nucleotide-free and GDP-bound forms - PubMed (original) (raw)
Crystal structure of a dynamin GTPase domain in both nucleotide-free and GDP-bound forms
H H Niemann et al. EMBO J. 2001.
Abstract
Dynamins form a family of multidomain GTPases involved in endocytosis, vesicle trafficking and maintenance of mitochondrial morphology. In contrast to the classical switch GTPases, a force-generating function has been suggested for dynamins. Here we report the 2.3 A crystal structure of the nucleotide-free and GDP-bound GTPase domain of Dictyostelium discoideum dynamin A. The GTPase domain is the most highly conserved region among dynamins. The globular structure contains the G-protein core fold, which is extended from a six-stranded beta-sheet to an eight-stranded one by a 55 amino acid insertion. This topologically unique insertion distinguishes dynamins from other subfamilies of GTP-binding proteins. An additional N-terminal helix interacts with the C-terminal helix of the GTPase domain, forming a hydrophobic groove, which could be occupied by C-terminal parts of dynamin not present in our construct. The lack of major conformational changes between the nucleotide-free and the GDP-bound state suggests that mechanochemical rearrangements in dynamin occur during GTP binding, GTP hydrolysis or phosphate release and are not linked to loss of GDP.
Figures
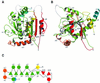
Fig. 1. Structure and topology of the GTPase domain of dynamin A. The G-protein core fold is shown in green; αA in yellow; the insertion comprising β2A, αB, β2B in red; αC′ and αC in orange; and the extension of α5 after the kink in blue. The asterisk marks the tip of the variable loop connecting β2 and β2A. Missing loops are in white. (A) Front view. The β-sheet is extended beyond β2 to a total of eight strands. (B) Side view. αA and αB pack against the sheet from different sides, while αC runs perpendicular to the β-strands. αA and the extension of α5 make contacts at the back of the β-sheet. β3 and β6 are not labeled as they are hidden behind β2 and α1, respectively. (C) Topology diagram. β-strands coming out of the plane of the paper are triangles with tip up, while those running into the plane are tip down. Figures 1A and B, 4 and 5 were produced using Molscript (Kraulis, 1991) and Raster3d (Merritt and Bacon, 1997).
Fig. 2. Structure-based sequence alignment of dynamin A from D.discoideum, human dynamin 1 and Ras. Rectangles indicate helices, arrows β-sheets and dashed lines disordered regions. Colors of secondary structure elements correspond to those in Figure 1. The consensus elements for GTP binding are boxed and labeled G1–G4. The position of the residues mutated in the temperature-sensitive alleles of Drosophila (_shi_ts1,2) and C.elegans (ky51) is marked. The asterisk marks the same residues as in Figure 1. Residues identical in at least two of the proteins are boxed in black; conservative substitutions are shaded in gray.
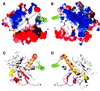
Fig. 3. Possible interaction site of the GTPase domain with the GED. The C-terminal helix of the myosin tag is shown in green with three hydrophobic side chains as a stick model. It binds into a groove of hydrophobic residues formed by helices αA and α5 of dynamin. The nucleotide is shown in yellow, and the asterisks mark the position of Asp74 and Asp75 in the loop connecting β2 and β2A. (A) Electrostatic surface potential of the dynamin A GTPase domain. Electrostatic potential is shown in red for negative charge, ramping through white at neutral charge to blue at positive charge. The two lines mark an exposed hydrophobic patch that runs from the groove formed by αA and α5 to the nucleotide-binding site. Disordered loops that might need stabilization by the C-terminal part of dynamin cluster around the nucleotide. The GED could occupy a position equivalent to that of the myosin helix and extend to the nucleotide, thereby covering the exposed hydrophobic patch. The insertion comprising β2A, αB and β2B is highly negatively charged. (B) The view from (A) rotated by 180°. The surface of helices αC and α5 is positively charged. (C and D) Ribbon diagrams of the same orientation as in (A) and (B). The topologically unique dynamin insertion is colored red, the C-terminal helix α5 is orange, and the ends of disordered loops are in cyan. The figure was produced with the Swiss-PDBViewer (Guex and Peitsch, 1997) and POV-Ray.
Fig. 4. Comparison of the nucleotide-binding site of empty and GDP-bound dynamin A with those of empty EF-G and GDP-bound Ras. (A) In nucleotide-free dynamin A, the side chain of Thr207 from the TKLD motif makes a hydrogen bond (dashed lines) to the carbonyl of Ser36 in the P-loop. Lys38 binds to residues from switch II (Asp138 and Leu139). (B) In GDP-bound dynamin A, Lys38 preserves its interactions with residues from switch II and does not bind to the β-phosphate. Lys208 binds the endocyclic oxygen of the ribose and Asp210 makes two hydrogen bonds with the base, while Thr207 does not bind the base. The coordination of the Mg2+ (magenta), which is usually octahedral in G-proteins [see (D)], is non-standard due to the disorder of the structural elements and water molecules (cyan) in this region. (C) In nucleotide-free EF-G, the P-loop Lys25 binds to residues from switch II, as in dynamin A. Asn137, equivalent to dynamin A Thr207, interacts with the side chain of Thr28 in the helix following the P-loop. (D) The canonical nucleotide-binding site of Ras-GDP. Lys16 binds to the β-phosphate. The interactions of Lys117 and Asp119 with the nucleotide correspond to those of Lys208 and Asp210 in dynamin A. Asn116 in Ras makes two hydrogen bonds, one to the carbonyl of Val14 in the P-loop and one to the base.
Fig. 5. Comparison of dynamin A Phe50 and Ras Phe28. Dynamin A is colored in orange and Ras in green. GDP is only shown for Ras. Gly47 at the end of helix α1 allows the switch I loop to take off at a different angle in dynamin A than in Ras, which has no glycine at the equivalent position. In dynamin A, Phe50 is buried by hydrophobic residues of the β-sheet (not shown) instead of stabilizing the base as Ras Phe28 does. The position of the Ras G5 motif (145SAK147) and of dynamin A Asn238 and Arg 239 is also shown.
Similar articles
- Crystal structure of the GTPase domain and the bundle signalling element of dynamin in the GDP state.
Anand R, Eschenburg S, Reubold TF. Anand R, et al. Biochem Biophys Res Commun. 2016 Jan 1;469(1):76-80. doi: 10.1016/j.bbrc.2015.11.074. Epub 2015 Nov 21. Biochem Biophys Res Commun. 2016. PMID: 26612256 - Crystal structure of the GDP-bound GTPase domain of Rab5a from Leishmania donovani.
Zohib M, Maheshwari D, Pal RK, Freitag-Pohl S, Biswal BK, Pohl E, Arora A. Zohib M, et al. Acta Crystallogr F Struct Biol Commun. 2020 Nov 1;76(Pt 11):544-556. doi: 10.1107/S2053230X20013722. Epub 2020 Oct 29. Acta Crystallogr F Struct Biol Commun. 2020. PMID: 33135673 Free PMC article. - Dynamin GTPase, a force-generating molecular switch.
Warnock DE, Schmid SL. Warnock DE, et al. Bioessays. 1996 Nov;18(11):885-93. doi: 10.1002/bies.950181107. Bioessays. 1996. PMID: 8939066 Review. - The three-dimensional model of Dictyostelium discoideum racE based on the human rhoA-GDP crystal structure.
Agarwal M, Nelson DJ, Larochelle DA. Agarwal M, et al. J Mol Graph Model. 2002 Aug;21(1):3-18. doi: 10.1016/s1093-3263(01)00137-1. J Mol Graph Model. 2002. PMID: 12413026 - Structure-function relationships of the G domain, a canonical switch motif.
Wittinghofer A, Vetter IR. Wittinghofer A, et al. Annu Rev Biochem. 2011;80:943-71. doi: 10.1146/annurev-biochem-062708-134043. Annu Rev Biochem. 2011. PMID: 21675921 Review.
Cited by
- Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission.
Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE. Mears JA, et al. Nat Struct Mol Biol. 2011 Jan;18(1):20-6. doi: 10.1038/nsmb.1949. Epub 2010 Dec 19. Nat Struct Mol Biol. 2011. PMID: 21170049 Free PMC article. - Structural basis for the allosteric interference of myosin function by reactive thiol region mutations G680A and G680V.
Preller M, Bauer S, Adamek N, Fujita-Becker S, Fedorov R, Geeves MA, Manstein DJ. Preller M, et al. J Biol Chem. 2011 Oct 7;286(40):35051-60. doi: 10.1074/jbc.M111.265298. Epub 2011 Aug 13. J Biol Chem. 2011. PMID: 21841195 Free PMC article. - Affinity Purification and Functional Characterization of Dynamin-Related Protein 1.
Clinton RW, Bauer BL, Mears JA. Clinton RW, et al. Methods Mol Biol. 2020;2159:41-53. doi: 10.1007/978-1-0716-0676-6_4. Methods Mol Biol. 2020. PMID: 32529362 Free PMC article. - A corkscrew model for dynamin constriction.
Mears JA, Ray P, Hinshaw JE. Mears JA, et al. Structure. 2007 Oct;15(10):1190-202. doi: 10.1016/j.str.2007.08.012. Structure. 2007. PMID: 17937909 Free PMC article. - Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic Vesicle Recycling and Implications in Neurological Diseases.
Prichard KL, O'Brien NS, Murcia SR, Baker JR, McCluskey A. Prichard KL, et al. Front Cell Neurosci. 2022 Jan 18;15:754110. doi: 10.3389/fncel.2021.754110. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115907 Free PMC article. Review.
References
- Amor J.C., Harrison,D.H., Kahn,R.A. and Ringe,D. (1994) Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature, 372, 704–708. - PubMed
- Bourne H.R., Sanders,D.A. and McCormick,F. (1991) The GTPase superfamily: conserved structure and molecular mechanism. Nature, 349, 117–127. - PubMed
- Brünger A.T. et al. (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr., D54, 905–921. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases