Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses - PubMed (original) (raw)
Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses
M Hartmann et al. EMBO J. 2001.
Abstract
The protein brain-derived neurotrophic factor (BDNF) has been postulated to be a retrograde or paracrine synaptic messenger in long-term potentiation and other forms of activity-dependent synaptic plasticity. Although crucial for this concept, direct evidence for the activity-dependent synaptic release of BDNF is lacking. Here we investigate secretion of BDNF labelled with green fluorescent protein (BDNF-GFP) by monitoring the changes in fluorescence intensity of dendritic BDNF-GFP vesicles at glutamatergic synaptic junctions of living hippocampal neurons. We show that high-frequency activation of glutamatergic synapses triggers the release of BDNF-GFP from synaptically localized secretory granules. This release depends on activation of postsynaptic ionotropic glutamate receptors and on postsynaptic Ca(2+) influx. Release of BDNF-GFP is also observed from extrasynaptic dendritic vesicle clusters, suggesting that a possible spatial restriction of BDNF release to specific synaptic sites can only occur if the postsynaptic depolarization remains local. These results support the concept of BDNF being a synaptic messenger of activity-dependent synaptic plasticity, which is released from postsynaptic neurons.
Figures
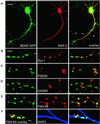
Fig. 1. Synaptic targeting of BDNF–GFP. Immunofluorescent detection of the dendritic marker protein MAP2 (A), the general presynaptic marker protein Synapsin I (B), the marker protein PSD95 for glutamatergic synapses (C) and the marker protein GAD65 for GABAergic synapses (D), respectively. (E) Identification of presynaptic terminals in living neurons using activity-dependent labelling with FM 4-64. In (A–E), each panel shows dendrites of a BDNF–GFP-expressing hippocampal neuron (left), the fluorescence image for the respective marker (middle) and the overlay of both (right). (F) Left: live staining of FM 4-64-labelled synaptic terminals (red) of a BDNF–GFP (green)-expressing neuron shows synaptic localization of BDNF–GFP (yellow). Middle: posthoc labelling of dendrites using MAP2 antibody (blue). The overlay on the right shows co-localization of the three signals in white. Scale bars: 10 µm (A), 4 µm (B–F). The arrowheads in (A) depict dendritic filopodia.
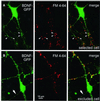
Fig. 2. Cell selection for BDNF–GFP release experiments. (A) BDNF–GFP fluorescence (left), FM 4-64 labelling of presynaptic terminals (middle) and merged pictures (right) of a typical hippocampal neuron selected for release experiments. All four soma-derived processes are dendrites, as is evident from the large diameter of the proximal processes and from the FM 4-64-labelled terminals approaching them. Arrowheads depict synaptic BDNF–GFP clusters used for release measurements. For these clusters, postsynaptic localization is very likely because a BDNF–GFP-containing axon is not present. (B) Sequence of images as in (A), but from a neuron that was excluded from release experiments: the thin, soma-derived process (arrow), which is not contacted by FM 4-64-labelled terminals, is the presumed axon of the cell and contains BDNF–GFP fluorescence. Such cells were excluded because of possible additional axonal localization of BDNF–GFP. Scale bar: 10 µm.
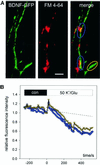
Fig. 3. Monitoring depolarization-induced synaptic secretion of BDNF–GFP as a decrease in fluorescence intensity. (A) Synaptic co-localization (yellow) of BDNF–GFP (green) with FM 4-64 (red). Monochrome images show original BDNF–GFP fluorescence at the time points indicated. Scale bar: 5 µm. (B) Relative change (standardized to t = 0, start of stimulation) of averaged BDNF–GFP fluorescence intensities of colour-coded regions of interest marked in (A) upon application of 50 mM K+ and 300 µM
l
-glutamate in physiological saline (2 mM Ca2+, 1 mM Mg2+). The dashed line is extrapolated from photobleaching observed during 5 min of control superfusion (see Materials and methods). (C) Time course of the average fluorescence decrease of BDNF–GFP clusters using the different stimulation solutions as indicated. All solutions contained 2 mM Ca2+ and 1 mM Mg2+.
dl
-APV (200 µM), DNQX (20 µM) and LY 341495 (100 µM) were added to one group of cells to inhibit ionotropic and metabotropic glutamate receptors. Tetanus toxin: BDNF–GFP-expressing neurons were pre-incubated with tetanus toxin (1 nM, 20 h) prior to recording. The asterisk represents the starting point of significant difference between ‘control’ and ‘K+/glu’ at the _p_-level indicated. (D) Release of BDNF–GFP was blocked in the absence of extracellular Ca2+ and by inhibiting VGCC with Ni2+ (100 µM) and Cd2+ (100 µM). Average change in fluorescence intensity of BDNF–GFP clusters from different groups of cells as indicated. Mg2+ concentration was 1 mM, except for ‘K+/Glu/0 Ca2+’ (3 mM Mg2+). The asterisk represents the starting point of significant difference between K+/Glu ± 2 mM Ca2+ at the _p_-level indicated. All traces were corrected for photobleaching by subtraction of the fluorescence decrease in negative controls (0 mM Ca2+, 3 mM Mg2+, 5 mM EGTA).
Fig. 4. Simultaneous release of BDNF–GFP from postsynaptic and extrasynaptic dendritic locations. (A) BDNF–GFP fluorescence (left), FM 4-64 labelling of presynaptic terminals (middle) and merged images (right) obtained from a dendritic branch of a transfected neuron. Synaptic (blue) and extrasynaptic (yellow) BDNF–GFP vesicle clusters are marked. Scale bar: 5 µm. (B) The time course of fluorescence decrease upon stimulation with K+/Glu for colour-coded regions shown in (A) reveals a comparable degree of BDNF–GFP release from synaptic and extrasynaptic clusters. Since vesicular axonal release is restricted to presynaptic terminals, which are absent from extrasynaptic BDNF–GFP clusters (marked in yellow), the release must have occurred from dendrites.
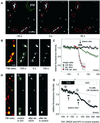
Fig. 5. Synaptic release of BDNF–GFP upon high-frequency synaptic stimulation. (A) Monochrome images (at the time points indicated) from a representative destaining experiment of FM 4-64-labelled presynaptic terminals using extracellular tetanic stimulation [800 pulses in 16 × 1 s bursts (2.5 s intervals) at 50 Hz]. pip., stimulation pipette. (B) Synaptic co-localization of BDNF–GFP clusters with FM 4-64 (left) and monochrome images of BDNF–GFP fluorescence before and after tetanic stimulation [same paradigm as in (A)]. Images in (A) and (B) are from different cells. (C) Average change in fluorescence intensity of the postsynaptic BDNF–GFP clusters marked in (B) (green triangles) versus results of a non-stimulated cell under identical conditions (black circles). Red squares show averaged release of FM 4–64 from the presynaptic terminals marked in (A). The arrow pointing to a black bar indicates the duration of stimulation. (D) Synaptic co-localization (yellow) of BDNF–GFP clusters (green) with FM 4–64 (red) of a cell subjected to two rounds of tetanic stimulation. Monochrome images show BDNF–GFP fluorescence prior to first tetanus in DNQX/APV (a), 250 s after first tetanus in D/A and immediately prior to the second tetanus (b), and 250 s after second tetanus in control (c). Note the stimulus-induced loss of fluorescence only between b and c. (E) Averaged data from eight cells as in (D). The labels a–c indicate time points of images shown in (D). Data are aligned with respect to the two tetani (at 0′ and 0; duration of tetani indicated by arrows pointing to black bars). The gap on the time axis accounts for slightly different intervals between the two tetani in individual cells (210–380 s). The dashed line is extrapolated from photobleaching during 5 min prior to first tetanus. The reversible block of BDNF–GFP release by APV and DNQX reveals that synaptic secretion of BDNF–GFP upon presynaptic tetanic stimulation depends on depolarization via postsynaptic glutamate receptors. Scale bars: 10 µm (A), 3 µm (B and D).
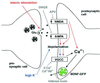
Fig. 6. Synaptic secretion of BDNF–GFP by different routes of postsynaptic depolarization. Tetanic presynaptic stimulation leads, via intermediate repetitive glutamate release, to postsynaptic depolarization and Ca2+ influx (red route). This depolarization can be blocked by ionotropic glutamate receptor antagonists (see Figure 5). In contrast, high K+ solution directly depolarizes the postsynaptic membrane (blue route), activates VGCCs, and can thus bypass intermediate glutamate release and activation of postsynaptic glutamate receptors (see Figure 3). The observed inhibition of BDNF release by blocking Ca2+ influx (0 Ca2+; Ni2+/Cd2+) and the independence of BDNF release from glutamate receptors (both during stimulation with high K+) demonstrate the direct dependence of BDNF secretion on postsynaptic Ca2+ influx. Likewise, since K+-induced postsynaptic depolarization is independent of glutamate release, the inhibition of high K+-induced BDNF secretion by tetanus toxin indicates that BDNF secretion depends on the fusion of secretory vesicles.
Similar articles
- Brain-derived neurotrophic factor increases inhibitory synapses, revealed in solitary neurons cultured from rat visual cortex.
Palizvan MR, Sohya K, Kohara K, Maruyama A, Yasuda H, Kimura F, Tsumoto T. Palizvan MR, et al. Neuroscience. 2004;126(4):955-66. doi: 10.1016/j.neuroscience.2004.03.053. Neuroscience. 2004. PMID: 15207329 - Activity- and BDNF-induced plasticity of miniature synaptic currents in ES cell-derived neurons integrated in a neocortical network.
Copi A, Jüngling K, Gottmann K. Copi A, et al. J Neurophysiol. 2005 Dec;94(6):4538-43. doi: 10.1152/jn.00155.2005. J Neurophysiol. 2005. PMID: 16293594 - Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging.
Lochner JE, Honigman LS, Grant WF, Gessford SK, Hansen AB, Silverman MA, Scalettar BA. Lochner JE, et al. J Neurobiol. 2006 May;66(6):564-77. doi: 10.1002/neu.20250. J Neurobiol. 2006. PMID: 16555239 - Activity-dependent dendritic secretion of brain-derived neurotrophic factor modulates synaptic plasticity.
Kuczewski N, Porcher C, Gaiarsa JL. Kuczewski N, et al. Eur J Neurosci. 2010 Oct;32(8):1239-44. doi: 10.1111/j.1460-9568.2010.07378.x. Epub 2010 Sep 30. Eur J Neurosci. 2010. PMID: 20880359 Review. - Neurotrophins and hippocampal synaptic transmission and plasticity.
Lu B, Chow A. Lu B, et al. J Neurosci Res. 1999 Oct 1;58(1):76-87. J Neurosci Res. 1999. PMID: 10491573 Review.
Cited by
- Multiple approaches to investigate the transport and activity-dependent release of BDNF and their application in neurogenetic disorders.
Hartmann D, Drummond J, Handberg E, Ewell S, Pozzo-Miller L. Hartmann D, et al. Neural Plast. 2012;2012:203734. doi: 10.1155/2012/203734. Epub 2012 Jun 6. Neural Plast. 2012. PMID: 22720171 Free PMC article. Review. - SNARE-Mediated Exocytosis in Neuronal Development.
Urbina FL, Gupton SL. Urbina FL, et al. Front Mol Neurosci. 2020 Aug 7;13:133. doi: 10.3389/fnmol.2020.00133. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32848598 Free PMC article. Review. - Propofol-induced changes in neurotrophic signaling in the developing nervous system in vivo.
Popic J, Pesic V, Milanovic D, Todorovic S, Kanazir S, Jevtovic-Todorovic V, Ruzdijic S. Popic J, et al. PLoS One. 2012;7(4):e34396. doi: 10.1371/journal.pone.0034396. Epub 2012 Apr 4. PLoS One. 2012. PMID: 22496799 Free PMC article. - Glucocorticoid affects dendritic transport of BDNF-containing vesicles.
Adachi N, Numakawa T, Nakajima S, Fukuoka M, Odaka H, Katanuma Y, Ooshima Y, Hohjoh H, Kunugi H. Adachi N, et al. Sci Rep. 2015 Aug 4;5:12684. doi: 10.1038/srep12684. Sci Rep. 2015. PMID: 26239075 Free PMC article. - Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling.
Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR. Neal AP, et al. Toxicol Sci. 2010 Jul;116(1):249-63. doi: 10.1093/toxsci/kfq111. Epub 2010 Apr 7. Toxicol Sci. 2010. PMID: 20375082 Free PMC article.
References
- Ahnert-Hilger G. and Bigalke,H. (1995) Molecular aspects of tetanus and botulinum neurotoxin poisoning. Prog. Neurobiol., 46, 83–96. - PubMed
- Angleson J.K., Cochilla,A.J., Kilic,G., Nussinovitch,I. and Betz,W.J. (1999) Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytotic events. Nature Neurosci., 2, 440–446. - PubMed
- Ankarcrona M., Dypbukt,J.M., Bonfoco,E., Zhivotovsky,B., Orrenius,S., Lipton,S.A. and Nicotera,P. (1995) Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron, 15, 961–973. - PubMed
- Berninger B. and Poo,M. (1996) Fast actions of neurotrophic factors. Curr. Opin. Neurobiol., 6, 324–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous