The trans Golgi network is lost from cells infected with African swine fever virus - PubMed (original) (raw)
The trans Golgi network is lost from cells infected with African swine fever virus
M McCrossan et al. J Virol. 2001 Dec.
Abstract
The cellular secretory pathway is important during the assembly and envelopment of viruses and also controls the transport of host proteins, such as cytokines and major histocompatibility proteins, that function during the elimination of viruses by the immune system. African swine fever virus (ASFV) encodes at least 26 proteins with stretches of hydrophobic amino acids suggesting entry into the secretory pathway (R. J. Yanez, J. M. Rodriguez, M. L. Nogal, L. Yuste, C. Enriquez, J. F. Rodriguez, and E. Vinuela, Virology 208:249-278, 1995). To predict how and where these potential membrane proteins function, we have studied the integrity of the secretory pathway in cells infected with ASFV. Remarkably, ASFV caused complete loss of immunofluorescence signal for the trans Golgi network (TGN) marker protein TGN46 and dispersed the AP1 TGN adapter complex. Loss of TGN46 signal was not due to degradation of TGN46, suggesting redistribution of TGN46 to other membrane compartments. ASFV markedly slowed transport of cathepsin D to lysosomes, demonstrating that loss of TGN structure correlated with loss of TGN function. ASFV shows a tropism for macrophages, and it is possible that ASFV compromises TGN function to augment the activity of viral membrane proteins or to suppress the function of host immunoregulatory proteins.
Figures
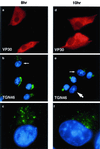
FIG. 1
The early effects of ASFV on the TGN. BSC40 cells were fixed 8 h (a to c) or 10 h (d to f) after infection with the Ba71v strain of ASFV. Samples were incubated with a monoclonal antibody specific for early viral protein vp30 (a and d) and a rabbit antibody specific for TGN46 (b and e). Cellular and viral DNA was visualized by DAPI (b and e). Antigens were visualized by second antibodies coupled to Alexa 488 or Alexa 594. Samples were viewed at ×60, and 0.2-μm digital sections were digitally deconvolved with Openlab software from Improvision. Panels b and e show a digital merge of the DAPI and TGN46 distributions. The small arrows indicate cells with disrupted TGN, which are shown at higher magnification in panels c and f. The large arrow indicates a cell lacking TGN signal.
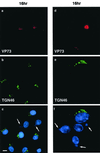
FIG. 2
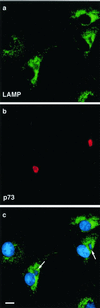
Effects of ASFV on TGN at 16 h postinfection. BSC40 cells were fixed 16 h after infection with the Ba71v strain of ASFV. Samples were incubated with a monoclonal antibody specific for the major capsid protein p73 (a and d) and a rabbit antibody specific for TGN46 (b and e). Cellular and viral DNA was visualized by DAPI (c and f). Antigens were visualized by second antibodies coupled to Alexa 488 or Alexa 594. Samples were viewed at ×60, and 0.2-μm digital sections were digitally deconvolved with Openlab software from Improvision. The bar in panel c represents 10 μm. Note that the cells in panels d, e, and f are shown at higher magnification. Panels c and f show a digital merge of the DAPI and TGN46 distributions. The arrows indicate cells with extranuclear DAPI staining of virus factories and a lack of TGN46 signal.
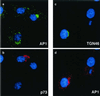
FIG. 3
Effects of ASFV on TGN adapter protein AP1. BSC40 cells were fixed 16 h after infection with the Ba71v strain of ASFV. Samples were incubated with a monoclonal antibody specific for the major capsid protein p73 and a rabbit antibody specific for AP1 (a and b) or a goat antibody specific for TGN46 and a rabbit antibody specific for AP1 (c and d). In each image, cellular and viral DNA was visualized by DAPI. Antigens were visualized by second antibodies coupled to Alexa 488 or Alexa 594. Samples were viewed at ×60, and 0.2-μm digital sections were digitally deconvolved with Openlab software from Improvision. Panels a and b compare the AP1 and p73 distributions, while panels c and d compare AP1 and TGN46.
FIG. 4
Effect of ASFV infection on transport of cathepsin D to lysosomes. Vero cells were pulse-labeled for 15 min with [35S]methionine and [35S]cysteine and then chased in complete media. At the indicated time points, cells were lysed and immunoprecipitated with an antibody specific for cathepsin D. One half of each immunoprecipitate was digested with endo H (+). Samples were analyzed by SDS-PAGE followed by autoradiography. The preproenzyme (53 kDa) and mature enzyme (31 kDa) are indicated. (Top panel) Control cells. (Bottom panel) Cells analyzed 16 h postinfection (pi) with ASFV.
FIG. 5
Loss of the TGN46 signal does not involve degradation of TGN46. The stability of TGN46 in Vero cells was determined by adding cycloheximide (10 μg/ml) to cell cultures. The cells were then lysed at the indicated times, and the levels of TGN46 were determined by Western blotting. For infected cells, cycloheximide was added 4 h after addition of virus to allow expression of early genes prior to the block in protein synthesis with cycloheximide. The addition of virus (ASFV ±) or cycloheximide (CHX ±) is indicated.
FIG. 6
Loss of the TGN46 signal does not require late ASFV gene expression. Vero cells were fixed 16 h after infection with the Ba71v strain of ASFV. (a to c) Control cells incubated without Ara-C. (d to f) Cells incubated with 50 μg of Ara-C per ml added 4 h postinfection. Cells were stained with antibodies specific for TGN46 (a and d), biotinylated antibody specific for the early ASFV protein p30 (b and e), and a monoclonal antibody binding the late structural protein, p73 (c and f). Antigens were visualized with secondary antibodies coupled to Alexa 488 (a and d), Alexa 594 (c and f), and avidin coupled to Cascade Blue (b and e). Arrows identify an infected cell.
FIG. 7
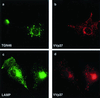
Effect of ASFV on lysosomes. BSC40 cells were fixed 16 h after infection with the Ba71v strain of ASFV and incubated with a monoclonal antibody (H4B4) specific for LAMP2 (a and c) and a rabbit antibody specific for viral capsid protein p73 (b). Cellular and viral DNA was visualized by DAPI (c). Antigens were visualized by second antibodies coupled to Alexa 488 or Alexa 594. Samples were viewed at ×60, and 0.2-μm digital sections were digitally deconvolved with Openlab software from Improvision. Panels c shows a digital merge of the DAPI and LAMP2 distributions. Bar, 10 μm. The arrows indicate extranuclear DAPI staining of viral DNA in virus factories.
FIG. 8
Effect of vaccinia virus on the TGN and lysosomes. BSC40 cells were fixed 16 h after infection with the VTF7 strain of vaccinia virus and incubated with a rabbit antibody specific for TGN46 (a) or monoclonal antibody H4B4 specific for LAMP2 (c). Vaccinia virus infection was detected with a rat antibody (15B6) that recognizes viral protein VV-p37 (b and d). Antigens were visualized by second antibodies coupled to Alexa 488 or Alexa 594.
Similar articles
- African swine fever virus causes microtubule-dependent dispersal of the trans-golgi network and slows delivery of membrane protein to the plasma membrane.
Netherton CL, McCrossan MC, Denyer M, Ponnambalam S, Armstrong J, Takamatsu HH, Wileman TE. Netherton CL, et al. J Virol. 2006 Nov;80(22):11385-92. doi: 10.1128/JVI.00439-06. Epub 2006 Sep 6. J Virol. 2006. PMID: 16956944 Free PMC article. - African swine fever virus infects macrophages, the natural host cells, via clathrin- and cholesterol-dependent endocytosis.
Galindo I, Cuesta-Geijo MA, Hlavova K, Muñoz-Moreno R, Barrado-Gil L, Dominguez J, Alonso C. Galindo I, et al. Virus Res. 2015 Mar 16;200:45-55. doi: 10.1016/j.virusres.2015.01.022. Epub 2015 Feb 3. Virus Res. 2015. PMID: 25662020 - A BIR motif containing gene of African swine fever virus, 4CL, is nonessential for growth in vitro and viral virulence.
Neilan JG, Lu Z, Kutish GF, Zsak L, Burrage TG, Borca MV, Carrillo C, Rock DL. Neilan JG, et al. Virology. 1997 Apr 14;230(2):252-64. doi: 10.1006/viro.1997.8481. Virology. 1997. PMID: 9143281 - African swine fever virus controls the host transcription and cellular machinery of protein synthesis.
Sánchez EG, Quintas A, Nogal M, Castelló A, Revilla Y. Sánchez EG, et al. Virus Res. 2013 Apr;173(1):58-75. doi: 10.1016/j.virusres.2012.10.025. Epub 2012 Nov 12. Virus Res. 2013. PMID: 23154157 Review. - Protein transport from the secretory to the endocytic pathway in mammalian cells.
Le Borgne R, Hoflack B. Le Borgne R, et al. Biochim Biophys Acta. 1998 Aug 14;1404(1-2):195-209. doi: 10.1016/s0167-4889(98)00057-3. Biochim Biophys Acta. 1998. PMID: 9714803 Review.
Cited by
- Legionella pneumophila Infection of Human Macrophages Retains Golgi Structure but Reduces O-Glycans.
Fu Y, Macwan V, Heineman RE, Terebiznik MR, Harrison RE. Fu Y, et al. Pathogens. 2022 Aug 12;11(8):908. doi: 10.3390/pathogens11080908. Pathogens. 2022. PMID: 36015029 Free PMC article. - Selective fragmentation of the trans-Golgi apparatus by Rickettsia rickettsii.
Aistleitner K, Clark T, Dooley C, Hackstadt T. Aistleitner K, et al. PLoS Pathog. 2020 May 18;16(5):e1008582. doi: 10.1371/journal.ppat.1008582. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32421751 Free PMC article. - African Swine Fever Virus Host-Pathogen Interactions.
Netherton CL, Shimmon GL, Hui JYK, Connell S, Reis AL. Netherton CL, et al. Subcell Biochem. 2023;106:283-331. doi: 10.1007/978-3-031-40086-5_11. Subcell Biochem. 2023. PMID: 38159232 - Phosphorylation of Golgi Peripheral Membrane Protein Grasp65 Is an Integral Step in the Formation of the Human Cytomegalovirus Cytoplasmic Assembly Compartment.
Rebmann GM, Grabski R, Sanchez V, Britt WJ. Rebmann GM, et al. mBio. 2016 Oct 4;7(5):e01554-16. doi: 10.1128/mBio.01554-16. mBio. 2016. PMID: 27703074 Free PMC article. - Unconventional Pathways of Secretion Contribute to Inflammation.
Daniels MJ, Brough D. Daniels MJ, et al. Int J Mol Sci. 2017 Jan 5;18(1):102. doi: 10.3390/ijms18010102. Int J Mol Sci. 2017. PMID: 28067797 Free PMC article. Review.
References
- Brookes S M, Sun H, Dixon L K, Parkhouse R M E. Characterisation of African swine fever virion proteins j5R and j13L: immunolocalization in virus particles and assembly sites. J Gen Virol. 1998;79:1179–1188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous