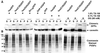N-terminal protein acylation confers localization to cholesterol, sphingolipid-enriched membranes but not to lipid rafts/caveolae - PubMed (original) (raw)
N-terminal protein acylation confers localization to cholesterol, sphingolipid-enriched membranes but not to lipid rafts/caveolae
J B McCabe et al. Mol Biol Cell. 2001 Nov.
Free PMC article
Abstract
When variably fatty acylated N-terminal amino acid sequences were appended to a green fluorescent reporter protein (GFP), chimeric GFPs were localized to different membranes in a fatty acylation-dependent manner. To explore the mechanism of localization, the properties of acceptor membranes and their interaction with acylated chimeric GFPs were analyzed in COS-7 cells. Myristoylated GFPs containing a palmitoylated or polybasic region colocalized with cholesterol and ganglioside GM(1), but not with caveolin, at the plasma membrane and endosomes. A dipalmitoylated GFP chimera colocalized with cholesterol and GM(1) at the plasma membrane and with caveolin in the Golgi region. Acylated GFP chimeras did not cofractionate with low-density caveolin-rich lipid rafts prepared with Triton X-100 or detergent-free methods. All GFP chimeras, but not full-length p62(c-yes) and caveolin, were readily solubilized from membranes with various detergents. These data suggest that, although N-terminal acylation can bring GFP to cholesterol and sphingolipid-enriched membranes, protein-protein interactions are required to localize a given protein to detergent-resistant membranes or caveolin-rich membranes. In addition to restricting acceptor membrane localization, N-terminal fatty acylation could represent an efficient means to enrich the concentration of signaling proteins in the vicinity of detergent-resistant membranes and facilitate protein-protein interactions mediating transfer to a detergent-resistant lipid raft core.
Figures
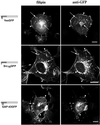
Figure 1
Colocalization of chimeric GFPs with free cholesterol in COS-7 cells. Cells transfected with plasmids expressing various GFP chimeras were fixed, permeabilized, and incubated with filipin to detect free cholesterol and rabbit anti-GFP antibody followed by FITC-conjugated anti-rabbit antibody to detect acylated GFPs. In the models beside the images: dark gray/blue box, appended acylation sequence; light gray/green box, GFP; small acyl chain, myristate; large acyl chain, palmitate; plus (+) signs, polybasic region. Arrowheads indicate areas of plasma membrane colocalization, and arrows indicate endosomal/Golgi colocalization. Bars, 10 μm.
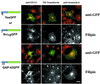
Figure 2
Acute cholesterol depletion alters intracellular localization of acylated GFP chimeras and GM1. COS-7 cells, grown in lipoprotein-deficient serum for 18–20 h, were acutely depleted of cholesterol with the use of 20 mM MβCD for 1 h at 37°C. (A) GFP chimeras were detected with the use of rabbit anti-GFP and FITC-conjugated donkey anti-rabbit secondary antibody. Free cholesterol was detected with filipin. (B) Ganglioside GM1 distribution detected with FITC-CTX. Arrowheads indicate areas of plasma membrane colocalization, and arrows indicate endosomal colocalization. Model descriptions of Figures 2–4 are as in Figure 1. (C) Viability of MβCD-treated COS-7 cells expressing YesGFP. Expressing cells were treated with vehicle, 20 mM MβCD for 1 h at 37°C, or 20 mM MβCD for 1 h followed by repletion with 30 μg/ml cholesterol:MβCD for 1 h at 37°C. Cellular viability was determined by MTT formazan production. Bar, 10 μm.
Figure 3
Triple colocalization of various chimeric GFPs with organelle markers and free cholesterol as detected by filipin in COS-7 cells. Colocalization of differentially acylated GFP chimeras (green) and organelle marker (red) distributions in transfected COS-7 cells can be seen in color. Filipin signal is shown in grayscale panels below corresponding color organelle colocalization panels. Bar, 10 μm.
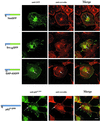
Figure 4
Colocalization of various chimeric GFPs with the ganglioside GM1. Colocalization of GM1 as detected with FITC-CTX (green) with acylated chimeric GFPs YesGFP, Src16GFP, and GAP-43GFP, as detected with the use of rabbit anti-GFP antibody followed by TR-conjugated donkey anti-rabbit antibody. Arrowheads indicate areas of plasma membrane colocalization, and arrows indicate endosomal/Golgi colocalization. Bars, 10 μm.
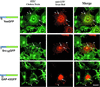
Figure 5
Colocalization of chimeric GFPs and endogenous p62c-yes PTK with caveolin in COS-7 cells. COS-7 cells were transfected with plasmids encoding various acylated GFP chimeras and processed for indirect immunofluorescence analysis 24 h after transfection. The GFP chimeras were detected by incubation with mouse anti-GFP antibody followed by FITC-conjugated anti-mouse antibody. Endogenous p62c-yes was detected with mouse anti-Yes antibody and FITC-conjugated donkey anti-mouse secondary antibody. The same cells were incubated with rabbit anti-caveolin antibody followed by TR-conjugated anti-rabbit secondary antibody. Arrowheads point to caveolin-GAP-43GFP Golgi colocalization, and arrows point to clusters of acylated GFP-containing structures and caveolin-containing structures. Bars, 10 μm.
Figure 6
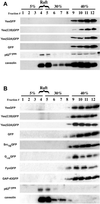
Caveolin-enriched lipid raft isolation with the use of detergent-based and detergent-free sucrose density gradient fractionation methods. (A) Caveolin-enriched lipid raft isolation with the use of a TX-100 at 4°C fractionation method. COS-7 cells transiently transfected with various acylated GFPs and GFP alone were subjected to subcellular fractionation after homogenization in buffer containing 1% TX-100. The distribution of acylated chimeric GFPs and endogenous caveolin are shown with the use of a discontinuous 5–40% sucrose gradient. Fractions were collected from the top of the gradient, separated by SDS-PAGE (12.5% acrylamide), and analyzed by immunoblotting and Coomassie staining of the immunoblot. Immunoblot analysis was done with rabbit anti-GFP antibody (1:2000) to detect GFP and the acylated chimeras, with anti-caveolin (1:4000) to detect endogenous caveolin, and with mouse anti-Yes antibody (1:1000) to detect endogenous p62c-yes PTK. (B) Caveolin enriched lipid raft isolation with the use of a sodium carbonate fractionation method. Transfected COS-7 cells were subjected to subcellular fractionation after homogenization in buffer containing 500 mM sodium carbonate, pH 11.0. The distribution of acylated chimeric GFPs and endogenous caveolin and p62c-yes PTK are shown. Fractions were collected and analyzed as above.
Figure 7
Solubilization of acylated chimeric GFPs expressed in COS-7 cells by 0.1 or 1% TX-100 and 60 mM OG at 4°C. COS-7 cells expressing various acylated and nonacylated GFP chimeras, and GFP alone, were solubilized in detergent at 4°C for 20 min and then separated into soluble (S) and resistant (P) fractions. Aliquots of soluble and resistant fractions were analyzed by immunoblotting with the use of rabbit anti-GFP and anti-caveolin antibodies. (A) Immunoblots show the distribution of the expressed proteins and endogenous caveolin in soluble and resistant fractions in the presence of indicated detergents. Plus signs indicate the type and concentration of detergent used to solubilize the cells. (B) Coomassie-stained PVDFs, corresponding to the immunoblots above, depict distribution of total cellular protein after detergent solubilization.
Figure 8
Solubilization kinetics of acylated GFPs, caveolin, and p62c-yes. COS-7 cells expressing various acylated and nonacylated GFP chimeras were separated into 4°C TX-100-soluble and -resistant fractions by incubating cell monolayers with 1% TX-100 for 0–20 min. The 1% TX-100 fractions containing solubilized total cellular proteins were rapidly removed from the tissue culture dishes and the detergent-resistant matrices solubilized with a 1× lysis buffer solution. These separated fractions were immunoblotted with rabbit anti-GFP (1:2000), anti-caveolin (1:4000), and mouse anti-Yes (1:1000) antibodies. (A) Immunoblots showing relative distributions of acylated GFPs, caveolin, and p62c-yes between TX-100-soluble and -resistant fractions. Markers, right, point to the position of migration of the indicated proteins. Below each immunoblot is a corresponding Coomassie-stained PVDF membrane showing the kinetic distribution of total cellular protein between TX-100-soluble and -resistant fractions. (B) Immunoblot showing the specificity of the three antibodies used in this analysis. YesGFP is being detected in the anti-GFP blot.
Figure 9
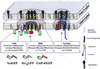
Model of fatty acylation-dependent membrane localization. The model depicts the localization of acylated GFPs with cholesterol and sphingolipid-enriched membrane microdomains (CSMs, dotted area). All three types of membrane association signal combinations (myristoylation plus palmitoylation: YesGFP; myristoylation plus polybasic region: Src16GFP; and dual palmitoylation plus polybasic region: GAP-43GFP) showed similar localization at the plasma membrane. Acylated full-length Yes PTK is shown in the cholesterol- and sphingolipid-enriched detergent-resistant membrane fraction (DRM, hatched area). Yes PTK is depicted in a DRM separate from DRMs containing caveolin, right (because Yes PTK and caveolin do not colocalize). Protein-protein interactions (represented by association with the unidentified orange protein) are believed to mediate Yes PTK association with the DRM core. For simplicity, lipid rafts present in endosomal or Golgi structures are not illustrated. Models of various lipid raft membrane constituents are shown in the legend at right.
Similar articles
- Functional roles for fatty acylated amino-terminal domains in subcellular localization.
McCabe JB, Berthiaume LG. McCabe JB, et al. Mol Biol Cell. 1999 Nov;10(11):3771-86. doi: 10.1091/mbc.10.11.3771. Mol Biol Cell. 1999. PMID: 10564270 Free PMC article. - P-Glycoprotein is localized in intermediate-density membrane microdomains distinct from classical lipid rafts and caveolar domains.
Radeva G, Perabo J, Sharom FJ. Radeva G, et al. FEBS J. 2005 Oct;272(19):4924-37. doi: 10.1111/j.1742-4658.2005.04905.x. FEBS J. 2005. PMID: 16176266 - Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae (review).
Hooper NM. Hooper NM. Mol Membr Biol. 1999 Apr-Jun;16(2):145-56. doi: 10.1080/096876899294607. Mol Membr Biol. 1999. PMID: 10417979 Review. - Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease.
Quest AF, Leyton L, Párraga M. Quest AF, et al. Biochem Cell Biol. 2004 Feb;82(1):129-44. doi: 10.1139/o03-071. Biochem Cell Biol. 2004. PMID: 15052333 Review.
Cited by
- Emerging Roles of YES1 in Cancer: The Putative Target in Drug Resistance.
Kook E, Chun KS, Kim DH. Kook E, et al. Int J Mol Sci. 2024 Jan 25;25(3):1450. doi: 10.3390/ijms25031450. Int J Mol Sci. 2024. PMID: 38338729 Free PMC article. Review. - The cytoplasmic domain of human immunodeficiency virus type 1 transmembrane protein gp41 harbors lipid raft association determinants.
Yang P, Ai LS, Huang SC, Li HF, Chan WE, Chang CW, Ko CY, Chen SS. Yang P, et al. J Virol. 2010 Jan;84(1):59-75. doi: 10.1128/JVI.00899-09. J Virol. 2010. PMID: 19793805 Free PMC article. - Rapsyn escorts the nicotinic acetylcholine receptor along the exocytic pathway via association with lipid rafts.
Marchand S, Devillers-Thiéry A, Pons S, Changeux JP, Cartaud J. Marchand S, et al. J Neurosci. 2002 Oct 15;22(20):8891-901. doi: 10.1523/JNEUROSCI.22-20-08891.2002. J Neurosci. 2002. PMID: 12388596 Free PMC article. - Plasma membrane microdomains: organization, function and trafficking.
Laude AJ, Prior IA. Laude AJ, et al. Mol Membr Biol. 2004 May-Jun;21(3):193-205. doi: 10.1080/09687680410001700517. Mol Membr Biol. 2004. PMID: 15204627 Free PMC article. Review. - Specificity of membrane binding of the neuronal protein NAP-22.
Epand RF, Maekawa S, Epand RM. Epand RF, et al. J Membr Biol. 2003 Jun 1;193(3):171-6. doi: 10.1007/s00232-003-2015-y. J Membr Biol. 2003. PMID: 12962277
References
- Anderson RGW. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. - PubMed
- Andersson S, Davis DL, Dahlback H, Jornvall H, Russell DW. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. - PubMed
- Arni S, Keilbaugh SA, Ostermeyer AG, Brown DA. Association of GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J Biol Chem. 1998;273:28478–28485. - PubMed
- Arreaza G, Melkonian KA, LaFevre-Bernt M, Brown DA. Triton X-100-resistant membrane complexes from cultured kidney epithelial cells contain the Src family protein tyrosine kinase p62yes. J Biol Chem. 1994;269:19123–19127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical