TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis - PubMed (original) (raw)
TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis
A R Pettit et al. Am J Pathol. 2001 Nov.
Abstract
There is considerable evidence that osteoclasts are involved in the pathogenesis of focal bone erosion in rheumatoid arthritis. Tumor necrosis factor-related activation-induced cytokine, also known as receptor activator of nuclear factor-kappaB ligand (TRANCE/RANKL) is an essential factor for osteoclast differentiation. In addition to its role in osteoclast differentiation and activation, TRANCE/RANKL also functions to augment T-cell dendritic cell cooperative interactions. To further evaluate the role of osteoclasts in focal bone erosion in arthritis, we generated inflammatory arthritis in the TRANCE/RANKL knockout mouse using a serum transfer model that bypasses the requirement for T-cell activation. These animals exhibit an osteopetrotic phenotype characterized by the absence of osteoclasts. Inflammation, measured by clinical signs of arthritis and histopathological scoring, was comparable in wild-type and TRANCE/RANKL knockout mice. Microcomputed tomography and histopathological analysis demonstrated that the degree of bone erosion in TRANCE/RANKL knockout mice was dramatically reduced compared to that seen in control littermate mice. In contrast, cartilage erosion was present in both control littermate and TRANCE/RANKL knockout mice. These results confirm the central role of osteoclasts in the pathogenesis of bone erosion in arthritis and demonstrate distinct mechanisms of cartilage destruction and bone erosion in this animal model of arthritis.
Figures
Figure 1.
TRANCE/RANKL KO and Ctl mice with serum transfer arthritis develop inflammation that is clinically and histologically similar. TRANCE/RANKL KO (KO) and Ctl mice were injected with K/B×N serum or PBS (experiments C and D only) and sacrificed at 12 to 13 days (experiments A and B) or 21 days (experiments C to E) after the initial serum injection. Arthritis was monitored by clinical index (A) and measurement of ankle thickness, represented as ankle thickening (B) and AUC(0-12)AT and MaxAT (C). Histopathological scoring of inflammation in H&E-stained sections (D) was performed as outlined in Table 1 ▶ .
Figure 2.
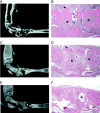
MicroCT and histological analysis of bone erosion in TRANCE/RANKL KO and Ctl mice. To assess bone erosion in TRANCE/RANKL KO and Ctl mice with serum transfer arthritis microCT images and histological sections were analyzed and correlated. Matched sagittal microCT images and H&E-stained sections for each sample were identified and compared: A and B, arthritic Ctl mouse, day 21; C and D, arthritic TRANCE/RANKL KO mouse, day 21; E and F, PBS-injected TRANCE/RANKL KO mouse, day 21. In A to C, asterisks indicate examples of significant erosion in Ctl mice, and the absence of erosion at the same anatomical location in TRANCE/RANKL KO mice in matched microCT images and H&E sections. In D and E, asterisks indicate the same anatomical location in the matched microCT image and H&E section. Arrows indicate sites of new bone formation. Original magnifications: ×4 (B, D, and F).
Figure 3.
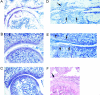
TRAP+ multinucleated cells are present in focal bone erosion in arthritic Ctl mice. H&E and TRAP histochemistry were performed in serial sections of arthritic Ctl and TRANCE/RANKL KO mice. A: H&E section illustrating focal bone erosion in an arthritic Ctl mouse sacrificed on day 21. B: Serial section stained for TRAP. Numerous multinucleated TRAP+ cells are present on the surface of the cortical and trabecular bone in areas of erosion. C: H&E section of an arthritic TRANCE/RANKL KO mouse sacrificed at day 21. D: Serial section stained for TRAP. Asterisks indicate the same anatomical site in A to D. E: High-power view of arthritic Ctl mouse demonstrating multinucleated (arrows) and occasional mononuclear TRAP+ cells in subchondral bone erosions. F and G: H&E-stained sections illustrating areas of irregular osteopetrotic bone present in both the arthritic (F) and PBS-injected (G) TRANCE/RANKL KO mice. Note that the inflamed tissue fills spaces to which it has direct access, including nutrient artery entry sites into bone. Original magnifications: ×10 (A–D, F, and G); ×40 (E).
Figure 4.
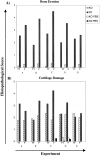
TRANCE/RANKL KO mice with serum transfer arthritis are protected from bone erosion. Histopathological scoring of bone erosion (A) and cartilage damage (B) were performed using the criteria outlined in Table 1 ▶ . The tibio-talar (TT) and forefoot (F) joints were scored separately and the average score for each site was compared with its matched pair. TRANCE/RANKL KO and Ctl mice were injected with K/B×N serum and sacrificed at days 12 to 13 (experiments A and B) or day 21 (experiments C to E) after the initial serum injection. In experiments C and D control TRANCE/RANKL KO (KO PBS) and Ctl (Ctl PBS) mice were injected with PBS. PBS injected control mice are not pictured in A because of the absence of bone erosion in these mice.
Figure 5.
Cartilage damage in TRANCE/RANKL KO mice. Cartilage damage remote from pannus tissue in Ctl and TRANCE/RANKL KO mice was assessed in toluidine blue-stained sections. Mild to marked cartilage proteoglycan, chondrocyte, and articular cartilage loss were observed in both the Ctl (A) and TRANCE/RANKL KO (B) arthritic mice. Toluidine blue-stained proteoglycan is present throughout articular cartilage in PBS-injected TRANCE/RANKL KO (C) and Ctl (data not shown) mice. Full-depth articular cartilage loss was observed in Ctl mice at sites of subchondral bone erosion where the scaffolding subchondral bony plate deep to articular cartilage was completely lost (D, arrows). These areas of subchondral bone erosion and associated full-depth articular cartilage loss were absent in TRANCE/RANKL KO mice (E, same anatomical location as illustrated in D). Areas of proteoglycan, chondrocyte, and cartilage loss ranging from superficial to deep zones were observed in TRANCE/RANKL KO mice (E, arrows). Destruction of articular cartilage directly adjacent to pannus was also observed in TRANCE/RANKL KO (F) and Ctl mice (data not shown). Original magnifications: ×10 (A–C); ×20 (D–F).
Similar articles
- Bone destruction in arthritis.
Gravallese EM. Gravallese EM. Ann Rheum Dis. 2002 Nov;61 Suppl 2(Suppl 2):ii84-6. doi: 10.1136/ard.61.suppl_2.ii84. Ann Rheum Dis. 2002. PMID: 12379632 Free PMC article. Review. - Increase in expression of receptor activator of nuclear factor kappaB at sites of bone erosion correlates with progression of inflammation in evolving collagen-induced arthritis.
Lubberts E, Oppers-Walgreen B, Pettit AR, Van Den Bersselaar L, Joosten LA, Goldring SR, Gravallese EM, Van Den Berg WB. Lubberts E, et al. Arthritis Rheum. 2002 Nov;46(11):3055-64. doi: 10.1002/art.10607. Arthritis Rheum. 2002. PMID: 12428250 - Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction.
Zwerina J, Hayer S, Tohidast-Akrad M, Bergmeister H, Redlich K, Feige U, Dunstan C, Kollias G, Steiner G, Smolen J, Schett G. Zwerina J, et al. Arthritis Rheum. 2004 Jan;50(1):277-90. doi: 10.1002/art.11487. Arthritis Rheum. 2004. PMID: 14730626 - RANKL protein is expressed at the pannus-bone interface at sites of articular bone erosion in rheumatoid arthritis.
Pettit AR, Walsh NC, Manning C, Goldring SR, Gravallese EM. Pettit AR, et al. Rheumatology (Oxford). 2006 Sep;45(9):1068-76. doi: 10.1093/rheumatology/kel045. Epub 2006 Feb 20. Rheumatology (Oxford). 2006. PMID: 16490750 - A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function.
Takahashi N, Udagawa N, Suda T. Takahashi N, et al. Biochem Biophys Res Commun. 1999 Mar 24;256(3):449-55. doi: 10.1006/bbrc.1999.0252. Biochem Biophys Res Commun. 1999. PMID: 10080918 Review.
Cited by
- RANK-Independent Osteoclast Formation and Bone Erosion in Inflammatory Arthritis.
O'Brien W, Fissel BM, Maeda Y, Yan J, Ge X, Gravallese EM, Aliprantis AO, Charles JF. O'Brien W, et al. Arthritis Rheumatol. 2016 Dec;68(12):2889-2900. doi: 10.1002/art.39837. Arthritis Rheumatol. 2016. PMID: 27563728 Free PMC article. - Short Peptides of Innate Immunity Protein Tag7 Inhibit the Production of Cytokines in CFA-Induced Arthritis.
Telegin GB, Chernov AS, Minakov AN, Balmasova IP, Romanova EA, Sharapova TN, Sashchenko LP, Yashin DV. Telegin GB, et al. Int J Mol Sci. 2022 Oct 17;23(20):12435. doi: 10.3390/ijms232012435. Int J Mol Sci. 2022. PMID: 36293292 Free PMC article. - FcRn is a CD32a coreceptor that determines susceptibility to IgG immune complex-driven autoimmunity.
Hubbard JJ, Pyzik M, Rath T, Kozicky LK, Sand KMK, Gandhi AK, Grevys A, Foss S, Menzies SC, Glickman JN, Fiebiger E, Roopenian DC, Sandlie I, Andersen JT, Sly LM, Baker K, Blumberg RS. Hubbard JJ, et al. J Exp Med. 2020 Oct 5;217(10):e20200359. doi: 10.1084/jem.20200359. J Exp Med. 2020. PMID: 32658257 Free PMC article. - Dynamic changes in O-GlcNAcylation regulate osteoclast differentiation and bone loss via nucleoporin 153.
Li YN, Chen CW, Trinh-Minh T, Zhu H, Matei AE, Györfi AH, Kuwert F, Hubel P, Ding X, Manh CT, Xu X, Liebel C, Fedorchenko V, Liang R, Huang K, Pfannstiel J, Huang MC, Lin NY, Ramming A, Schett G, Distler JHW. Li YN, et al. Bone Res. 2022 Jul 26;10(1):51. doi: 10.1038/s41413-022-00218-9. Bone Res. 2022. PMID: 35879285 Free PMC article. - Osteoclastogenesis and arthritis.
Maruotti N, Grano M, Colucci S, d'Onofrio F, Cantatore FP. Maruotti N, et al. Clin Exp Med. 2011 Sep;11(3):137-45. doi: 10.1007/s10238-010-0117-2. Epub 2010 Nov 11. Clin Exp Med. 2011. PMID: 21069419 Review.
References
- Leisen JCC, Duncan H, Riddle JM, Pitchford WC: The erosive front: a topographic study of the junction between the pannus and the subchondral plate in the macerated rheumatoid metacarpal head. J Rheumatol 1988, 15:17-22 - PubMed
- Bromley M, Woolley DE: Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum 1984, 27:968-975 - PubMed
- Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, Goldring SR: Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum 2000, 43:250-258 - PubMed
- Hummel KM, Petrow PK, Franz JK, Muller-Ladner U, Aicher WK, Gay RE, Bromme D, Gay S: Cysteine proteinase cathepsin K mRNA is expressed in synovium of patients with rheumatoid arthritis and is detected at sites of synovial bone destruction. J Rheumatol 1998, 25:1887-1894 - PubMed
- Hattersley G, Chambers TJ: Generation of osteoclastic function in mouse bone marrow cultures: multinuclearity and tartrate-resistant acid phosphatase are unreliable markers for osteoclastic differentiation. Endocrinology 1989, 124:1689-1696 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases