Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells - PubMed (original) (raw)
Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells
C Yeaman et al. J Cell Biol. 2001.
Abstract
Sec6/8 complex regulates delivery of exocytic vesicles to plasma membrane docking sites, but how it is recruited to specific sites in the exocytic pathway is poorly understood. We identified an Sec6/8 complex on trans-Golgi network (TGN) and plasma membrane in normal rat kidney (NRK) cells that formed either fibroblast- (NRK-49F) or epithelial-like (NRK-52E) intercellular junctions. At both TGN and plasma membrane, Sec6/8 complex colocalizes with exocytic cargo protein, vesicular stomatitis virus G protein (VSVG)-tsO45. Newly synthesized Sec6/8 complex is simultaneously recruited from the cytosol to both sites. However, brefeldin A treatment inhibits recruitment to the plasma membrane and other treatments that block exocytosis (e.g., expression of kinase-inactive protein kinase D and low temperature incubation) cause accumulation of Sec6/8 on the TGN, indicating that steady-state distribution of Sec6/8 complex depends on continuous exocytic vesicle trafficking. Addition of antibodies specific for TGN- or plasma membrane-bound Sec6/8 complexes to semiintact NRK cells results in cargo accumulation in a perinuclear region or near the plasma membrane, respectively. These results indicate that Sec6/8 complex is required for several steps in exocytic transport of vesicles between TGN and plasma membrane.
Figures
Figure 1.
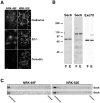
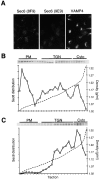
Sec6/8 complex is associated with membranes in fibroblastic and epithelial-like NRK cells. (A) Immunofluorescent staining of NRK-49F and NRK-52E cells with antibodies to E/P-cadherin, ZO-1, or occludin. Cells were fixed with 4% paraformaldehyde then extracted with 1% Triton X-100. (B) Immunodetection of Sec6, Sec8, and Exo70 in detergent lysates (50 μg/lane) from NRK-49F (F) and NRK-52E (E) cells. Protein standards indicated are myosin (205 kD), β-galactosidase (116 kD), phosphorylase b (97 kD), bovine serum albumin (66 kD), and egg albumin (45 kD). (C) Separation of membrane-bound and cytosolic Sec6/8 complexes from NRK-49F and NRK-52E cells in 30% (wt/vol) iodixanol gradients. Bar, 20 μm.
Figure 2.
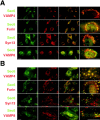
Sec6/8 complex colocalizes with adhesion junction proteins and actin filaments at cell–cell contact sites in NRK-49F and NRK-52E cells. NRK-49F cells (A) or NRK-52E cells (B) were fixed with 4% paraformaldehyde before or after extraction with 1% Triton X-100, as indicated. Sec6 (green) distribution was compared with that of ZO-1, ZO-2, occludin, E- or P-cadherin, α-catenin, and filamentous actin (red). Top right of A and B, as well as bottom of A and B show magnified views of cell–cell contacts. Bars, 20 μm.
Figure 3.
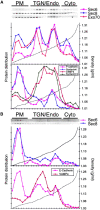
Fractionation of NRK-49F and NRK-52E cells in iodixanol gradients. Separation of Sec6, Sec8, Exo70, E- or P-cadherin, VAMP4, syntaxin4, and syntaxin13 from NRK-49F (A) or NRK-52E (B) cell homogenates in 10, 20, and 30% step-iodixanol gradients. PM represents fractions containing the major peak of plasma membrane proteins (E- or P-cadherin, syntaxin4), TGN/Endo represents fractions containing the major peaks of TGN (VAMP4) and early/recycling endosomes (syntaxin13), and Cyto represents fractions that did not contain any membrane protein marker.
Figure 4.
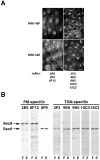
Sec6/8 complex epitopes are differentially accessible at plasma membrane cell–cell contacts and TGN/endosomes. (A) NRK-49F and NRK-52E cells were fixed with 4% paraformaldehyde then permeabilized with 0.075% saponin. Left panels show cells stained with anti-Sec8 mAb 2E9, which recognizes Sec8 bound to plasma membrane cell–cell contacts. Right panels show cells that were stained with anti-Sec6 mAb 10C3, which recognizes Sec6 bound to TGN/endosomes. Staining observed with other anti-Sec6 mAbs (3F3, 9E9, 9H5, and 15C2) was identical to that with mAb 10C3. (B) Detection of Sec6 and 8 in detergent extracts (50 μg/lane) of NRK-49F (F) and NRK-52E (E) with different monoclonal antibodies. Bar, 20 μm.
Figure 5.
Immunofluorescent staining of Sec6 and TGN/endosome markers in cells treated with and without nocodazole. NRK-49F cells were cultured in the absence (A) or presence (B) of 20 μg/ml nocodazole for 2 h. Cells were fixed with 4% paraformaldehyde, then permeabilized with 0.075% saponin. Sec6 staining (mAb 9E9) was compared with that of VAMP4, furin, syntaxin13, or VAMP8. Far right panels in each row of images show magnified views of perinuclear membranes from individual cells. Bars, 20 μm.
Figure 6.
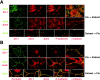
Immunofluorescent staining of Sec6 compared with endocytic and exocytic cargo. (A) NRK-49F cells were labeled with Texas red transferrin as described in Materials and methods. Following internalization for 5 or 30 min, cells were fixed, permeabilized, and stained for Sec6 (mAb 9H5). (B) NRK-49F cells were transiently transfected with plasmid encoding ts-G-GFP, and incubated under conditions to arrest this protein in either the ER or TGN, as described in Materials and methods. Cells were then fixed, permeabilized, and stained for Sec6 (mAb 9H5). Bars, (A) 8 μm; (B) 5 μm.
Figure 7.
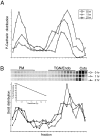
Metabolic pulse-chase analysis of P-cadherin and Sec8 in NRK-49F cells. 35S-methionine/cysteine distribution of 35S-methionine/cysteine–labeled P-cadherin (A) and Sec8 (B) in 10–20–30% step iodixanol gradients at different chase times. Plots are normalized to scale of 0 h time point. PM, TGN/Endo, and Cyto were defined as described in the legend to Fig. 3.
Figure 8.
Effect of BFA on steady-state binding and recruitment of newly synthesized Sec8 to plasma membrane and TGN. (A) NRK-49F cells were incubated in 3 μg/ml BFA for 5 min, fixed, permeabilized, and stained with anti-Sec6 mAbs 8F9 or 9E9 to visualize plasma membrane and TGN Sec6/8 complexes, respectively. Cells stained with mAb 9E9 were costained with AntiVAMP4 antibodies to visualize TGN. (B) NRK-49F cells were incubated in medium containing 5 μg/ml BFA for 30 min. Cell homogenates were mixed with 10, 20, and 30% iodixanol, layered step-wise in centrifuge tubes, and centrifuged at 350,000 g for 3 h. Presence of Sec8 in each gradient fraction was assayed by SDS-PAGE followed by immunoblotting with specific antibodies. (C) NRK-49F cells were incubated in medium lacking methionine/cysteine and containing 5 μg/ml BFA for 30 min then metabolically labeled with 35S-methionine/cysteine in the same medium for 60 min. Cell homogenates were fractionated as in B, and Sec8 was immunoprecipitated from each gradient fraction. PM, TGN, and Cyto were defined as described in the legend to Fig. 3.
Figure 9.
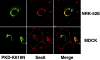
Immunofluorescent staining of Sec6 in cells following transient expression of PKD-K618N. NRK-52E and MDCK cells were transiently transfected with plasmid encoding GFP-PKD-K618N according to Materials and methods. MDCK cells were subsequently incubated at 19°C for 2 h. Cells were fixed, permeabilized, and stained for Sec6 (mAb 9H5). Bar, 5 μm.
Figure 10.
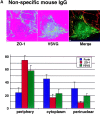
Requirement for plasma membrane- and TGN-bound Sec6/8 complex in exocytosis of ts-G-GFP in NRK-52E cells. NRK-52E cells were transiently transfected with plasmid encoding ts-G-GFP, incubated under conditions to arrest this protein in TGN, and then permeabilized with Digitonin as described in Materials and methods. Protein transport to the plasma membrane was reconstituted in the presence of nonspecific IgG (A) or anti-Sec6/8 antibodies directed against epitopes expressed at the plasma membrane (B) or TGN (C). Amounts of ts-G-GFP, ZO-1, and furin associated with perinuclear, cytoplasmic, or peripheral regions of ∼15 cells were quantified as described in Materials and methods. Representative analyses are shown above histograms of data from all analyses. (D) A gallery of representative images of semiintact NRK-52E cells in which TGN to plasma membrane transport of ts-G-GFP (green) was reconstituted in the presence of either control antibodies (left) or anti-PM Sec6/8 antibodies (right) and compared with the distribution of the plasma membrane-localized ZO-1 (red). Bars, 5 μm.
Figure 10.
Requirement for plasma membrane- and TGN-bound Sec6/8 complex in exocytosis of ts-G-GFP in NRK-52E cells. NRK-52E cells were transiently transfected with plasmid encoding ts-G-GFP, incubated under conditions to arrest this protein in TGN, and then permeabilized with Digitonin as described in Materials and methods. Protein transport to the plasma membrane was reconstituted in the presence of nonspecific IgG (A) or anti-Sec6/8 antibodies directed against epitopes expressed at the plasma membrane (B) or TGN (C). Amounts of ts-G-GFP, ZO-1, and furin associated with perinuclear, cytoplasmic, or peripheral regions of ∼15 cells were quantified as described in Materials and methods. Representative analyses are shown above histograms of data from all analyses. (D) A gallery of representative images of semiintact NRK-52E cells in which TGN to plasma membrane transport of ts-G-GFP (green) was reconstituted in the presence of either control antibodies (left) or anti-PM Sec6/8 antibodies (right) and compared with the distribution of the plasma membrane-localized ZO-1 (red). Bars, 5 μm.
Figure 10.
Requirement for plasma membrane- and TGN-bound Sec6/8 complex in exocytosis of ts-G-GFP in NRK-52E cells. NRK-52E cells were transiently transfected with plasmid encoding ts-G-GFP, incubated under conditions to arrest this protein in TGN, and then permeabilized with Digitonin as described in Materials and methods. Protein transport to the plasma membrane was reconstituted in the presence of nonspecific IgG (A) or anti-Sec6/8 antibodies directed against epitopes expressed at the plasma membrane (B) or TGN (C). Amounts of ts-G-GFP, ZO-1, and furin associated with perinuclear, cytoplasmic, or peripheral regions of ∼15 cells were quantified as described in Materials and methods. Representative analyses are shown above histograms of data from all analyses. (D) A gallery of representative images of semiintact NRK-52E cells in which TGN to plasma membrane transport of ts-G-GFP (green) was reconstituted in the presence of either control antibodies (left) or anti-PM Sec6/8 antibodies (right) and compared with the distribution of the plasma membrane-localized ZO-1 (red). Bars, 5 μm.
Figure 10.
Requirement for plasma membrane- and TGN-bound Sec6/8 complex in exocytosis of ts-G-GFP in NRK-52E cells. NRK-52E cells were transiently transfected with plasmid encoding ts-G-GFP, incubated under conditions to arrest this protein in TGN, and then permeabilized with Digitonin as described in Materials and methods. Protein transport to the plasma membrane was reconstituted in the presence of nonspecific IgG (A) or anti-Sec6/8 antibodies directed against epitopes expressed at the plasma membrane (B) or TGN (C). Amounts of ts-G-GFP, ZO-1, and furin associated with perinuclear, cytoplasmic, or peripheral regions of ∼15 cells were quantified as described in Materials and methods. Representative analyses are shown above histograms of data from all analyses. (D) A gallery of representative images of semiintact NRK-52E cells in which TGN to plasma membrane transport of ts-G-GFP (green) was reconstituted in the presence of either control antibodies (left) or anti-PM Sec6/8 antibodies (right) and compared with the distribution of the plasma membrane-localized ZO-1 (red). Bars, 5 μm.
Similar articles
- Ganglioside GD3 traffics from the trans-Golgi network to plasma membrane by a Rab11-independent and brefeldin A-insensitive exocytic pathway.
Crespo PM, Iglesias-Bartolomé R, Daniotti JL. Crespo PM, et al. J Biol Chem. 2004 Nov 12;279(46):47610-8. doi: 10.1074/jbc.M407181200. Epub 2004 Aug 31. J Biol Chem. 2004. PMID: 15339909 - Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells.
Beronja S, Laprise P, Papoulas O, Pellikka M, Sisson J, Tepass U. Beronja S, et al. J Cell Biol. 2005 May 23;169(4):635-46. doi: 10.1083/jcb.200410081. Epub 2005 May 16. J Cell Biol. 2005. PMID: 15897260 Free PMC article. - Effects of hypertonic and sodium-free medium on transport of a membrane glycoprotein along the secretory pathway in cultured mammalian cells.
Docherty PA, Snider MD. Docherty PA, et al. J Cell Physiol. 1991 Jan;146(1):34-42. doi: 10.1002/jcp.1041460106. J Cell Physiol. 1991. PMID: 1990018 - Targeting vesicles to specific sites on the plasma membrane: the role of the sec6/8 complex.
Hsu SC, Hazuka CD, Foletti DL, Scheller RH. Hsu SC, et al. Trends Cell Biol. 1999 Apr;9(4):150-3. doi: 10.1016/s0962-8924(99)01516-0. Trends Cell Biol. 1999. PMID: 10203793 Review.
Cited by
- The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons.
Dupraz S, Grassi D, Bernis ME, Sosa L, Bisbal M, Gastaldi L, Jausoro I, Cáceres A, Pfenninger KH, Quiroga S. Dupraz S, et al. J Neurosci. 2009 Oct 21;29(42):13292-301. doi: 10.1523/JNEUROSCI.3907-09.2009. J Neurosci. 2009. PMID: 19846717 Free PMC article. - The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains.
Fölsch H, Pypaert M, Maday S, Pelletier L, Mellman I. Fölsch H, et al. J Cell Biol. 2003 Oct 27;163(2):351-62. doi: 10.1083/jcb.200309020. J Cell Biol. 2003. PMID: 14581457 Free PMC article. - Membrane association and functional regulation of Sec3 by phospholipids and Cdc42.
Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. Zhang X, et al. J Cell Biol. 2008 Jan 14;180(1):145-58. doi: 10.1083/jcb.200704128. J Cell Biol. 2008. PMID: 18195105 Free PMC article. - Direct trafficking pathways from the Golgi apparatus to the plasma membrane.
Stalder D, Gershlick DC. Stalder D, et al. Semin Cell Dev Biol. 2020 Nov;107:112-125. doi: 10.1016/j.semcdb.2020.04.001. Epub 2020 Apr 13. Semin Cell Dev Biol. 2020. PMID: 32317144 Free PMC article. Review. - Epithelial cell surface polarity: the early steps.
Nejsum LN, Nelson WJ. Nejsum LN, et al. Front Biosci (Landmark Ed). 2009 Jan 1;14(3):1088-98. doi: 10.2741/3295. Front Biosci (Landmark Ed). 2009. PMID: 19273117 Free PMC article. Review.
References
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1987. Current Protocols in Molecular Biology. Vol. 1. John Wiley and Sons, New York. 86–102.
- Brennwald, P., B. Kearns, K. Champion, S. Keranen, V. Bankaitis, and P. Novick. 1994. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 79:245–258. - PubMed
- Cereijido, M., J. Valdes, L. Shoshani, and R.G. Contreras. 1998. Role of tight junctions in establishing and maintaining cell polarity. Annu. Rev. Physiol. 60:161–177. - PubMed
- Delgrossi, M.H., L. Breuza, C. Mirre, P. Chavrier, and A. Le Bivic. 1997. Human syntaxin 3 is localized apically in human intestinal cells. J. Cell Sci. 110:2207–2214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous