Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure - PubMed (original) (raw)
Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure
M J Burne et al. J Clin Invest. 2001 Nov.
Abstract
Leukocytes have been implicated in the pathogenesis of ischemic acute renal failure (ARF), but the roles of the individual cell types involved are largely unknown. Recent indirect evidence suggests that T cells may play an important role in a murine model of ARF. In the current study, we found that mice deficient in T cells (nu/nu mice) are both functionally and structurally protected from postischemic renal injury. Reconstitution of nu/nu mice with wild-type T cells restored postischemic injury. We then analyzed the contribution of the individual T cell subsets to postischemic injury and found that mice deficient in CD4(+) T cells, but not mice deficient in CD8(+) T cells, were significantly protected from ARF. Direct evidence for a pathophysiologic role of the CD4(+) T cell was obtained when reconstitution of CD4-deficient mice with wild-type CD4(+) T cells restored postischemic injury. In addition, adoptive transfers of CD4(+) T cells lacking either the costimulatory molecule CD28 or the ability to produce IFN-gamma were inadequate to restore injury phenotype. These results demonstrate that the CD4(+) T cell is an important mediator of ischemic ARF, and targeting this cell may yield novel therapies.
Figures
Figure 1
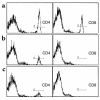
Flow cytometry confirmation of the absence or presence of CD4+ and/or CD8+ T cells. (a) CD4+ and CD8+ T cells are absent from spleens obtained from nu/nu mice. (b) After nu/nu mice were adoptively transferred with a T cell population from a wild-type control mouse, both CD4+ and CD8+ T cells were detected in spleens. The reconstitution of these cells in nu/nu mice ranged between 2 and 6%. (c) CD4-deficient mice show an absence of CD4+ T cells, but a normal complement of CD8+ T cells when compared with a wild-type control (not shown). (d) CD4-reconstituted CD4-deficient mice show a CD4+ population of approximately 2%.
Figure 2
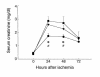
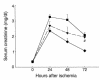
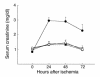
T cell–deficient mice are functionally protected from postischemic renal injury. SCr was significantly reduced at 24 and 48 hours (*P < 0.05) after IRI in nu/nu mice (circles) compared with SCr in wild-type control mice with IRI (squares). The injury phenotype was restored when nu/nu mice were adoptively transferred with wild-type T cells (triangles). Reconstituted nu/nu mice show a significantly higher SCr at 24 and 48 hours after ischemia (#P < 0.05) compared with nu/nu mice. In all experiments, SCr (mg/dl) was measured from tail blood samples obtained from animals at 0 (preischemia), 24, 48, and 72 hours after moderate ischemia (30 minutes of bilateral clamping). T cell–transferred mice received 15 × 106 purified T cells (obtained from wild-type control mice) intraperitoneally 3 weeks prior to ischemic injury.
Figure 3
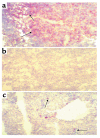
Histological assessment of tubular injury at 72 hours after ischemia in T cell–deficient and T cell–reconstituted mice. (a) Normal wild-type mouse kidney that has not undergone IRI. (b) Wild-type kidney 72 hours after ischemia demonstrates extensive tubular damage. (c) nu/nu postischemic kidney shows a significant reduction in renal structural injury. (d) In contrast, T cell–reconstituted nu/nu mice show a return of postischemic renal injury with structural damage similar to that seen in the wild-type kidney. ×50.
Figure 4
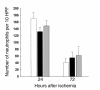
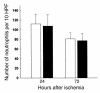
Neutrophil infiltration into the postischemic kidney at 24 and 72 hours after ischemia in T cell–deficient and T cell–reconstituted mice compared with wild-type control mice. At 24 and 72 hours after ischemia there was no significant difference in the number of infiltrating neutrophils between wild-type mice (white bars), nu/nu mice (black bars), and T cell–reconstituted nu/nu mice (gray bars). n = 6 per group. HPF, high-powered fields.
Figure 5
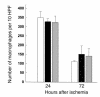
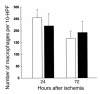
Macrophages infiltrating into the postischemic kidney at 72 hours after ischemia in T cell–deficient and T cell–reconstituted mice compared with wild-type control mice. At 72 hours after ischemia there was no significant difference in the number of infiltrating macrophages between wild-type mice (white bars), nu/nu mice (black bars), and T cell–reconstituted nu/nu mice (gray bars). n = 6 per group.
Figure 6
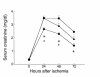
CD4-deficient mice are functionally protected from renal injury compared with CD8-deficient mice. CD4-deficient mice (diamonds) show significantly reduced SCr at all timepoints compared with wild-type mice (squares) (*P < 0.05) and with CD8-deficient mice (triangles) (#P < 0.05) at 24 and 48 hours after renal IRI.
Figure 7
Adoptive transfer of CD4+ T cells into CD4-deficient mice restores injury phenotype. CD4-deficient mice that have been reconstituted with wild-type CD4+ T cells (triangles, dashed line) have a return of the injury phenotype, with SCr values similar to wild-type control mice (squares). A significant increase in SCr in CD4-reconstituted mice was seen at 72 hours after ischemia compared with CD4-deficient mice (diamonds) (#P < 0.05). CD4-reconstituted mice received 5–10 × 106 purified T cells (obtained from wild-type control mice) intraperitoneally 3 weeks prior to ischemic injury.
Figure 8
Histological assessment of tubular injury at 24 hours after ischemia in CD4-deficient and CD8-deficient mice. (a) Normal wild-type mouse kidney that has not undergone IRI. (b) At 24 hours after ischemia, there is severe tubular damage in the wild-type control kidney. However, the CD4-deficient mouse kidney shows a significant reduction in tubular injury (c) compared with the wild-type control. In contrast, the CD8-deficient mouse kidney (d) shows tubular injury similar to that seen in the wild-type control kidney. ×50.
Figure 9
Neutrophils infiltrating into the postischemic kidney at 24 and 72 hours after ischemia in CD4-deficient and wild-type control mice. At 24 and 72 hours after ischemia, there is no significant difference seen in the number of infiltrating neutrophils between wild-type mice (open bars) and CD4-deficient mice (filled bars). n = 6 per group.
Figure 10
Macrophages infiltrating into the postischemic kidney at 24 and 72 hours after ischemia in CD4-deficient and wild-type control mice. At 24 and 72 hours after ischemia, there was no significant difference seen in the amount of infiltrating macrophages between wild-type mice (open bars) and CD4-deficient mice (filled bars). n = 6 per group.
Figure 11
CD4+ T cells infiltrating into the postischemic kidney. (a) Positively stained wild-type spleen showing the presence of CD4+ T cells. This is represented by brown rings surrounding positive cells. (b) Wild-type (no IRI) kidney stained with an antibody for CD4. (c) A 24-hour postischemic kidney obtained from a wild-type control mouse, showing a small infiltration of CD4+ T cells. ×50.
Figure 12
Flow cytometry confirmation of adoptive transfer of CD28-deficient and IFN-γ–deficient CD4+ T cells into T cell–deficient mice. (a) Wild-type spleen showing normal numbers of cells stained positively for CD4 (15.8%) and CD8 (13.1%). (b) nu/nu mice were reconstituted with CD4+ T cells (5.5% positive stained) from mice deficient in CD28. (c) nu/nu mice were reconstituted with CD4+ T cells (4% positive stained) from mice deficient in IFN-γ.
Figure 13
Adoptive transfer with CD4+ T cells from mice deficient in either CD28 or IFN-γ does not return the injury phenotype. nu/nu mice (diamonds) show significantly reduced SCr at all timepoints when compared with wild-type mice (circles). nu/nu mice reconstituted with CD4+ T cells from mice deficient in either CD28 (squares) or IFN-γ (triangles) also show significantly reduced SCr at all timepoints compared with wild-type control mice.
Similar articles
- A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity.
Liu M, Chien CC, Burne-Taney M, Molls RR, Racusen LC, Colvin RB, Rabb H. Liu M, et al. J Am Soc Nephrol. 2006 Mar;17(3):765-74. doi: 10.1681/ASN.2005010102. Epub 2006 Feb 15. J Am Soc Nephrol. 2006. PMID: 16481417 - Peripheral CD4 T-cell depletion is not sufficient to prevent ischemic acute renal failure.
Faubel S, Ljubanovic D, Poole B, Dursun B, He Z, Cushing S, Somerset H, Gill RG, Edelstein CL. Faubel S, et al. Transplantation. 2005 Sep 15;80(5):643-9. doi: 10.1097/01.tp.0000173396.07368.55. Transplantation. 2005. PMID: 16177639 - Lung T lymphocyte trafficking and activation during ischemic acute kidney injury.
Lie ML, White LE, Santora RJ, Park JM, Rabb H, Hassoun HT. Lie ML, et al. J Immunol. 2012 Sep 15;189(6):2843-51. doi: 10.4049/jimmunol.1103254. Epub 2012 Aug 10. J Immunol. 2012. PMID: 22888136 - Regulatory T cells modulate postischemic neovascularization.
Zouggari Y, Ait-Oufella H, Waeckel L, Vilar J, Loinard C, Cochain C, Récalde A, Duriez M, Levy BI, Lutgens E, Mallat Z, Silvestre JS. Zouggari Y, et al. Circulation. 2009 Oct 6;120(14):1415-25. doi: 10.1161/CIRCULATIONAHA.109.875583. Epub 2009 Sep 21. Circulation. 2009. PMID: 19770391 - T lymphocytes and acute kidney injury: update.
Martina MN, Noel S, Bandapalle S, Hamad AR, Rabb H. Martina MN, et al. Nephron Clin Pract. 2014;127(1-4):51-5. doi: 10.1159/000363719. Epub 2014 Sep 24. Nephron Clin Pract. 2014. PMID: 25343821 Free PMC article. Review.
Cited by
- Lymphocytes and innate immune cells in acute kidney injury and repair.
Lee K, Jang HR, Rabb H. Lee K, et al. Nat Rev Nephrol. 2024 Dec;20(12):789-805. doi: 10.1038/s41581-024-00875-5. Epub 2024 Aug 2. Nat Rev Nephrol. 2024. PMID: 39095505 Review. - A Novel Predictive Model for Acute Kidney Injury Following Surgery of the Aorta.
Chen M, Zhao S, Chen P, Zhao D, Wang L, Chen Z. Chen M, et al. Rev Cardiovasc Med. 2024 Feb 4;25(2):54. doi: 10.31083/j.rcm2502054. eCollection 2024 Feb. Rev Cardiovasc Med. 2024. PMID: 39077356 Free PMC article. - Protective effects of pioglitazone in renal ischemia-reperfusion injury (RIRI): focus on oxidative stress and inflammation.
Golmohammadi M, Ivraghi MS, Hasan EK, Huldani H, Zamanian MY, Rouzbahani S, Mustafa YF, Al-Hasnawi SS, Alazbjee AAA, Khalajimoqim F, Khalaj F. Golmohammadi M, et al. Clin Exp Nephrol. 2024 Oct;28(10):955-968. doi: 10.1007/s10157-024-02525-3. Epub 2024 Jun 27. Clin Exp Nephrol. 2024. PMID: 38935212 Review. - Lymphocyte-to-Red Blood Cell Ratio-The Guide Star of Acute Coronary Syndrome Prognosis.
Jercălău CE, Andrei CL, Brezeanu LN, Darabont RO, Guberna S, Catană A, Lungu MD, Ceban O, Sinescu CJ. Jercălău CE, et al. Healthcare (Basel). 2024 Jun 16;12(12):1205. doi: 10.3390/healthcare12121205. Healthcare (Basel). 2024. PMID: 38921319 Free PMC article. - Double-negative T cells have a reparative role after experimental severe ischemic acute kidney injury.
Lee K, Gharaie S, Kurzhagen JT, Newman-Rivera AM, Arend LJ, Noel S, Rabb H. Lee K, et al. Am J Physiol Renal Physiol. 2024 Jun 1;326(6):F942-F956. doi: 10.1152/ajprenal.00376.2023. Epub 2024 Apr 18. Am J Physiol Renal Physiol. 2024. PMID: 38634135
References
- Molitoris BA. Ischemic acute renal failure: exciting times at our fingertips. Curr Opin Nephrol Hypertens. 1998;7:405–406. - PubMed
- Humes HD. Acute renal failure: prevailing challenges and prospects for the future. Kidney Int. 1995;50(Suppl.):S26–S32. - PubMed
- Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. - PubMed
- Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333:333–336. - PubMed
- Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51:1463–1468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous