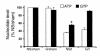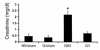Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury - PubMed (original) (raw)
Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury
K J Kelly et al. J Clin Invest. 2001 Nov.
Abstract
Ischemic injury to the kidney is characterized in part by nucleotide depletion and tubular cell death in the form of necrosis or apoptosis. Recently, we linked anoxia-induced apoptosis in renal cell cultures specifically to the depletion of GTP. We therefore hypothesized that enhancing GTP repletion in vivo might protect function by reducing apoptosis in postischemic tubules. Male C57 black mice (the "I" group of animals) underwent bilateral renal artery clamp for 32 minutes to induce ischemia and then received either normal saline ("NS") or guanosine ("G"). After 1 hour of reperfusion, renal GTP levels in NS/I were reduced to nearly half of those in sham operated mice, whereas these levels were nearly unchanged in G/I mice. Morphologic examination of tubular injury revealed no significant differences between the two groups. However, there was a significant reduction in the number of apoptotic tubular cells in the medulla in the G/I group as compared with the NS/I group. At 24 hours, creatinine was significantly elevated in the NS/I group, compared to the G/I group. We conclude that guanosine protects against renal ischemic injury by replenishing GTP stores and preventing tubular apoptosis.
Figures
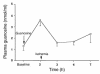
Figure 1
Effects of intraperitoneal guanosine on plasma guanosine levels. Animals received 30 mg/kg guanosine intraperitoneally 2 hours before bilateral renal ischemia/reperfusion. Blood was collected at various time points and plasma guanosine levels determined by HPLC as detailed in Methods. Values are means ± SE (n = 3).
Figure 2
Effects of guanosine on renal nucleotide levels during reperfusion. Values are means ± SE. Mice received normal saline (NS, n = 5) or guanosine (G, n = 5) 30 mg/kg intraperitoneally, 2 hours before 32-minute bilateral renal ischemia (I) or sham surgery. Renal tissues were obtained from each kidney 1 hour after surgery and processed for nucleotide determination (*P < 0.02, when GTP in NS/I was compared with GTP in G/I).
Figure 3
Effects of guanosine and enhanced GTP recovery on renal histology at 24 hours. Mice received normal saline (NS) or guanosine (G) 30 mg/kg intraperitoneally 2 hours before renal ischemia (I) or sham surgery. Representative H&E-stained sections from sham, NS/I, and G/I 24 hours after surgery are shown. Compared with the sham group, sections from both NS/I and G/I show patchy tubular dilatation, necrosis, and extensive cast formation. DAPI (blue) and FITC-phalloidin (green) staining show significant loss and/or disruption of the apical actin rim in both NS/I and G/I as compared with sham.
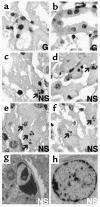
Figure 4
Effects of guanosine and enhanced GTP recovery on renal cell apoptosis at 24 hours. Mice were treated as detailed in legend of Figure 2. Kidney sections were obtained at 24 hours and costained with TUNEL and DAPI. (a) A negative control (NC; TdT omitted) and (b) a DNase-treated positive control (PC) for TUNEL. All TUNEL-positive nuclei had bright green fluorescence. (c) A representative field showing the medulla from NS/I group. Extensive TUNEL-positive staining is seen and is localized primarily to the tubular epithelium (dark green autofluorescence). (d) A representative field from the medulla of the G/I group showing lack of TUNEL-positive staining. (e) A magnification of TUNEL-positive nuclei from NS/I mice, with only the DAPI channel turned on. ×60. Nuclei are condensed, dysmorphic, and show heavy staining. Inset shows a single nucleus. ×120. Typical apoptotic fragmentation of chromatin into four small bodies is seen. (f) A magnification of TUNEL-negative nuclei from G/I mice. ×60. They show lack of condensation and faint DAPI staining.
Figure 5
Morphologic characterization of apoptosis by light and electron microscopy. Mice received normal saline (NS) or guanosine (G) 30 mg/kg intraperitoneally 2 hours before renal ischemia or sham surgery. Representative H&E-stained sections 24 hours after ischemia from the guanosine-treated group are shown (a and b). Nuclei with predominantly necrotic features are observed. They have degraded chromatin without formation of discrete, membrane-bound fragments. (c, d, e, and f) From the NS-treated group. Classic apoptotic bodies with dense condensation and fragmentation of chromatin into discrete fragments are seen (arrows). (g and h) Electron microscopic photomicrographs of sections from the normal saline group show clear apoptotic (g) and necrotic (h) morphologies.
Figure 6
Quantitative evaluation of TUNEL-positive nuclei in cortex and medulla. Values are means ± SE and represent number of TUNEL-positive nuclei expressed as percentage of total number of nuclei. Mice were treated as detailed in the legend of Figure 2. Kidney sections were obtained at 24 hours and processed for TUNEL and DAPI staining as detailed in Methods. Number of fields counted: 20 for G/I, 35 for NS/I, 8 for G/sham, and 8 for NS/sham. (*P < 0.01 when medullary TUNEL-positive nuclei in NS/I were compared to all other groups).
Figure 7
Distribution of TUNEL-positive nuclei among various nephronal segments. (a) A 5-μm thick section from NS/I mice stained with TUNEL and DAPI. The TUNEL positive nuclei were pseudocolored white instead of green to allow clear distinction from the green FITC-phalloidin. (b) A 5-μm section immediately contiguous to the one shown in a is stained with the actin marker FITC-phalloidin (bright green) to identify proximal tubules (P). Distal tubules (D) show minimal or no staining. The section was costained with anti-THP Ab and a Cy5-conjugated secondary Ab (red). This is predominantly a marker for Henle’s loop segments (LH). (c) An overlay of a and b allows the localization of TUNEL-positive nuclei to various tubular segments. (d) A quantitative estimate of the distribution of TUNEL-positive nuclei normalized to total number of DAPI-positive nuclei for each particular tubular segment or lumen. Other refers to interstitial or vascular TUNEL-positive nuclei.
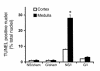
Figure 8
Effects of guanosine and enhanced renal GTP levels on renal function at 24 hours. Values are means ± SE. Creatinine levels at 24 hours are shown after administration of normal saline (NS) or guanosine (G) 2 hours before sham surgery or bilateral renal ischemia. Mean creatinine was significantly higher in the NS/I mice than in the other groups. (*P < 0.01, n = 6 for NS/I and G/I groups).
Figure 9
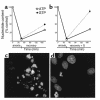
Effects of guanosine on nucleotide levels and apoptosis after chemical anoxia recovery in LLC-PK1 cells. Values are means ± SE. (a) LLC-PK1 cells were treated for 45 minutes with 0.1 μM antimycin A in depleted media followed by recovery for 2 hours. Nucleotides were measured at 45 minutes of depletion (n = 7) and at 2 hours of recovery (n = 6). (b) Cells were treated identically except for the addition of 200 μM guanosine to the recovery medium (n = 6). (c and d) Representative fields of confocal microscopic images of LLC-PK1 cells at 24 hours after recovery from chemical anoxia. Cells were costained with Hoechst 33342 and propidium iodide as detailed in Methods. In c, cells recovered in regular media. Apoptotic features such as condensation and fragmentation of chromatin is seen in most cells in the field. In d, cells recovered in the presence of 200 μM guanosine. Most nuclei showed normal morphology, and only few had apoptotic features.
Comment in
- Guanine nucleotides and acute renal failure.
Weinberg JM, Venkatachalam MA. Weinberg JM, et al. J Clin Invest. 2001 Nov;108(9):1279-81. doi: 10.1172/JCI14320. J Clin Invest. 2001. PMID: 11696571 Free PMC article. No abstract available.
Similar articles
- P53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: protective role of a p53 inhibitor.
Kelly KJ, Plotkin Z, Vulgamott SL, Dagher PC. Kelly KJ, et al. J Am Soc Nephrol. 2003 Jan;14(1):128-38. doi: 10.1097/01.asn.0000040596.23073.01. J Am Soc Nephrol. 2003. PMID: 12506145 - Apoptosis in ischemic renal injury: roles of GTP depletion and p53.
Dagher PC. Dagher PC. Kidney Int. 2004 Aug;66(2):506-9. doi: 10.1111/j.1523-1755.2004.761_7.x. Kidney Int. 2004. PMID: 15253698 Review. - Aprotinin improves kidney function and decreases tubular cell apoptosis and proapoptotic signaling after renal ischemia-reperfusion.
Kher A, Meldrum KK, Hile KL, Wang M, Tsai BM, Turrentine MW, Brown JW, Meldrum DR. Kher A, et al. J Thorac Cardiovasc Surg. 2005 Sep;130(3):662-9. doi: 10.1016/j.jtcvs.2005.02.035. J Thorac Cardiovasc Surg. 2005. PMID: 16153910 - Suramin promotes recovery from renal ischemia/reperfusion injury in mice.
Zhuang S, Lu B, Daubert RA, Chavin KD, Wang L, Schnellmann RG. Zhuang S, et al. Kidney Int. 2009 Feb;75(3):304-11. doi: 10.1038/ki.2008.506. Epub 2008 Oct 8. Kidney Int. 2009. PMID: 18843260 - Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury.
Hamar P, Song E, Kökény G, Chen A, Ouyang N, Lieberman J. Hamar P, et al. Proc Natl Acad Sci U S A. 2004 Oct 12;101(41):14883-8. doi: 10.1073/pnas.0406421101. Epub 2004 Oct 4. Proc Natl Acad Sci U S A. 2004. PMID: 15466709 Free PMC article.
Cited by
- Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism.
El-Achkar TM, McCracken R, Rauchman M, Heitmeier MR, Al-Aly Z, Dagher PC, Wu XR. El-Achkar TM, et al. Am J Physiol Renal Physiol. 2011 Apr;300(4):F999-1007. doi: 10.1152/ajprenal.00621.2010. Epub 2011 Jan 12. Am J Physiol Renal Physiol. 2011. PMID: 21228114 Free PMC article. - Induction of heat shock protein 70 inhibits ischemic renal injury.
Wang Z, Gall JM, Bonegio RG, Havasi A, Hunt CR, Sherman MY, Schwartz JH, Borkan SC. Wang Z, et al. Kidney Int. 2011 Apr;79(8):861-70. doi: 10.1038/ki.2010.527. Epub 2011 Jan 26. Kidney Int. 2011. PMID: 21270764 Free PMC article. - Targeted deletion of p53 in the proximal tubule prevents ischemic renal injury.
Ying Y, Kim J, Westphal SN, Long KE, Padanilam BJ. Ying Y, et al. J Am Soc Nephrol. 2014 Dec;25(12):2707-16. doi: 10.1681/ASN.2013121270. Epub 2014 May 22. J Am Soc Nephrol. 2014. PMID: 24854277 Free PMC article. - Stability and autolysis of cortical neurons in post-mortem adult rat brains.
Sheleg SV, Lobello JR, Hixon H, Coons SW, Lowry D, Nedzved MK. Sheleg SV, et al. Int J Clin Exp Pathol. 2008 Jan 1;1(3):291-9. Int J Clin Exp Pathol. 2008. PMID: 18784829 Free PMC article. - Pathogenesis of acute kidney injury: foundation for clinical practice.
Kinsey GR, Okusa MD. Kinsey GR, et al. Am J Kidney Dis. 2011 Aug;58(2):291-301. doi: 10.1053/j.ajkd.2011.02.385. Epub 2011 May 6. Am J Kidney Dis. 2011. PMID: 21530035 Free PMC article. Review.
References
- Kelly KJ, Molitoris BA. Acute renal failure in the new millennium: time to consider combination therapy. Semin Nephrol. 2000;20:4–19. - PubMed
- Star R. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. - PubMed
- Dubose TD, Jr, et al. Acute renal failure in the 21st century: recommendations for management and outcomes assessment. Am J Kidney Dis. 1997;29:793–799. - PubMed
- Safirstein R. Endothelin: the yin and yang of ischemic acute renal failure. Kidney Int. 2001;59:1590–1591. - PubMed
- Goligorsky MS. Endothelial cell dysfunction and nitric oxide synthase. Kidney Int. 2000;58:1360–1376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous