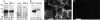Differential roles of CD36 and alphavbeta5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium - PubMed (original) (raw)
Differential roles of CD36 and alphavbeta5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium
S C Finnemann et al. J Exp Med. 2001.
Abstract
Retinal pigment epithelial (RPE) cells employ alphavbeta5 integrin and CD36 receptors to phagocytose photoreceptor outer segment fragments (OS). We explored special properties of RPE phagocytosis to identify the contribution of CD36 to RPE phagocytosis measuring effects of CD36 antibodies on OS binding and internalization kinetics. Early, CD36 antibodies had no effect on OS binding or internalization. Both control and CD36 antibody treated RPE initiated internalization approximately 2 hours after OS challenge. Later, bivalent CD36 IgG accelerated OS engulfment while monovalent Fab fragments inhibited engulfment. Cross-linking Fab fragments restored the accelerating activity of intact IgG. Strikingly, antibodies were effective even if added to OS already bound by RPE. alphavbeta5 blocking antibody reduced OS binding equally well in the presence of CD36 antibodies but CD36 antibodies accelerated internalization of remaining bound OS. Furthermore, CD36 ligation at either apical or basal RPE surface partially substituted for soluble factors that are required for internalization but not for binding of OS at the RPE apical surface. Our results demonstrate that CD36 ligation is necessary and sufficient to activate the OS internalization mechanism of RPE. They suggest that CD36 acts as a signaling molecule in postbinding steps of RPE phagocytosis independently of the OS binding receptor alphavbeta5 integrin.
Figures
Figure 1.
CD36 protein expression by stable human and rat-derived RPE derived cell lines. (A) Comparative immunoblotting of detergent extracts containing 20 μg total cellular protein followed by detection with rat CD36 antiserum shows that rat RPE-J cell lysates (RPE-J) contain ∼80% of the CD36 protein detected in lysates of RPE prepared from adult rat eyes (adult). Bands are specific for CD36 immunization, since they are absent in Western blot analysis probed with preimmune IgG. (B) Comparative immunoblotting of detergent extracts containing 100 μg total cellular protein followed by detection with human CD36 antiserum reveals the presence of CD36 protein of the expected molecular size of ∼88 kD in human ARPE-19 cells (ARPE-19) but not in human Bowes melanoma cells (Bowes), which are known not to express CD36 (reference 11). (C) Immunofluorescence labeling of paraformaldehyde fixed ARPE-19 cells with human CD36 mAb FA6–152 shows CD36 signals localizing to the RPE cell surface. (D) FA6–152 signals are specific since they are absent in parallel samples stained with nonimmune IgG.
Figure 2.
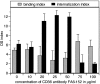
Concentration dependent effect of human CD36Ab FA6–152 on OS binding and internalization by ARPE-19 cells. Confluent, differentiated monolayers of ARPE-19 cells were challenged with FITC-labeled OS in the presence of 50 μg/ml nonimmune mouse IgG or increasing concentrations of FA6–152 as indicated for 5 h. Total (bound plus internalized) OS and internal (not quenched by Trypan's blue) OS were measured using fluorescence scanning and used to calculate the OS binding index (gray bars) and the OS internalization index (black bars) for each condition. The value of each bar represents the average OS index ± SD of four independent experiments. OS binding differences between samples were not statistically significant (P = 0.1). Student's t test analysis determined significant differences in OS internalization in the presence of 20–100 μg/ml FA6–152 as compared with control conditions (P < 0.001).
Figure 3.
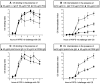
Effects of stimulating and inhibiting concentrations of CD36 Ab on the kinetics of OS binding and internalization by human ARPE-19 and rat RPE-J cells. Differentiated ARPE-19 cells were challenged for different periods of time up to 5 h with FITC-OS in the presence of 50 μg/ml nonimmune mouse IgG (•), 50 μg/ml FA6–152 (▾), and 100 μg/ml FA6–152 (▪). For all time points, binding indices (A) and internalization indices (B) were determined as described for Fig. 2 and plotted against the time after OS challenge. Differentiated monolayers of rat RPE-J cells were challenged with FITC-OS as described for ARPE-19 cells in the presence of IgG fractions of preimmune serum (•) or rat CD36 antiserum (x), both at 50 μg/ml. Binding and internalization indices were determined as described for ARPE-19 cells and plotted against the time of OS challenge in (C) and (D), respectively. Values given are average OS index ± SD of three independent experiments for ARPE-19 cells and of five independent experiments for RPE-J cells. n.i., nonimmune.
Figure 4.
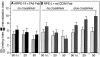
Inhibition but not stimulation of OS internalization by monovalent Fab fractions of CD36 Abs. 5 h of challenge with FITC-OS of human (A) and rat (B) RPE was followed by fluorescence scanning to calculate binding and internalization indices as described in Fig. 2. Light bars represent the binding index, dark bars the internalization index for each condition. Values in A and B represent averages ± SD of three independent experiments. (A) The presence of Fab fractions of FA6–152 IgG at 20 and 50 μg/ml, marked 20 and 50, respectively, did not significantly alter the amount of OS bound or internalized by ARPE-19 cells. Control cells received Fab fragments of nonimmune IgG at 50 μg/ml. (B) Fab fractions of rat CD36 IgG at 50 μg/ml, marked 50, decreased the OS internalization index of rat RPE-J cells to 54% of the index of cell receiving preimmune Fab fractions (Student's t test P < 0.005). Changes in bound OS were not significant. Cross-linking of CD36 Fab at 20 and 50 μg/ml, marked 20 and 50, using goat anti–rabbit Fab IgG at 10 μg/ml reversed the effect of CD36 Fab alone. Using Student's t test, these changes were significant with P < 0.005. n.i., nonimmune.
Figure 5.
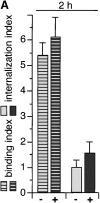
Addition of CD36Ab after OS binding to RPE is necessary and sufficient to increase the rate of OS internalization by RPE, independently of the OS binding receptor αvβ5. Differentiated monolayers of RPE-J cells were challenged for 2 h with FITC-OS in the presence of 50 μg/ml preimmune IgG or 50 μg/ml rat CD36 IgG. After 2 h, some samples were fixed to determine binding and internalization indices as shown in A. Striped bars, OS binding; filled bars, OS internalization; −, preimmune IgG control; +, CD36 IgG. (B) Identical samples as in A were washed with assay buffer 2 h after OS addition to remove unbound OS and further incubated in presence of either preimmune (−) or CD36 IgG (+). B shows the internalization indices of these samples determined 4 h after initial OS challenge (2 h after the removal of unbound OS). (C) We repeated the experiments shown in A and B but added αvβ5 inhibiting Ab P1F6 to all samples during the 2 h of OS challenge. P1F6 reduced OS binding at 2 h equally well in the presence of preimmune IgG, (−, dark striped bars) or of CD36 IgG (+, light striped bars). Internalization indices for both conditions were insignificant after 2 h (2 h, dark and light filled bars). Samples that were incubated with OS and P1F6 for 2 h were washed and incubated for another 2 h in the presence of preimmune (−) or CD36 IgG (+). Internalization indices that were determined after a total time of incubation of 4 h demonstrated that CD36 IgG accelerated internalization of OS prebound in the presence of P1F6 (4 h; −, preimmune IgG, +, CD36 IgG added after removal of unbound OS at 2 h). Note that the y axis scale in C is different from that in A and B. Values in A–C represent average OS indices ± SD (n = 3). (B and C). Statistical evaluation using Student's t test determined P < 0.001 for all changes compared with the appropriate control condition that were discussed as significant in the text. Note that all cells regardless of treatment completed internalization of prebound OS within 6 h after initial OS challenge (data not shown). Between the removal of unbound OS at 2 h and the termination of the experiment, the total amount of OS detected in each sample remained constant (data not shown). Thus, surface-bound OS did not dissociate from RPE cells and RPE cells did not degrade phagocytosed OS between 2 and 6 h after initial OS challenge.
Figure 5.
Addition of CD36Ab after OS binding to RPE is necessary and sufficient to increase the rate of OS internalization by RPE, independently of the OS binding receptor αvβ5. Differentiated monolayers of RPE-J cells were challenged for 2 h with FITC-OS in the presence of 50 μg/ml preimmune IgG or 50 μg/ml rat CD36 IgG. After 2 h, some samples were fixed to determine binding and internalization indices as shown in A. Striped bars, OS binding; filled bars, OS internalization; −, preimmune IgG control; +, CD36 IgG. (B) Identical samples as in A were washed with assay buffer 2 h after OS addition to remove unbound OS and further incubated in presence of either preimmune (−) or CD36 IgG (+). B shows the internalization indices of these samples determined 4 h after initial OS challenge (2 h after the removal of unbound OS). (C) We repeated the experiments shown in A and B but added αvβ5 inhibiting Ab P1F6 to all samples during the 2 h of OS challenge. P1F6 reduced OS binding at 2 h equally well in the presence of preimmune IgG, (−, dark striped bars) or of CD36 IgG (+, light striped bars). Internalization indices for both conditions were insignificant after 2 h (2 h, dark and light filled bars). Samples that were incubated with OS and P1F6 for 2 h were washed and incubated for another 2 h in the presence of preimmune (−) or CD36 IgG (+). Internalization indices that were determined after a total time of incubation of 4 h demonstrated that CD36 IgG accelerated internalization of OS prebound in the presence of P1F6 (4 h; −, preimmune IgG, +, CD36 IgG added after removal of unbound OS at 2 h). Note that the y axis scale in C is different from that in A and B. Values in A–C represent average OS indices ± SD (n = 3). (B and C). Statistical evaluation using Student's t test determined P < 0.001 for all changes compared with the appropriate control condition that were discussed as significant in the text. Note that all cells regardless of treatment completed internalization of prebound OS within 6 h after initial OS challenge (data not shown). Between the removal of unbound OS at 2 h and the termination of the experiment, the total amount of OS detected in each sample remained constant (data not shown). Thus, surface-bound OS did not dissociate from RPE cells and RPE cells did not degrade phagocytosed OS between 2 and 6 h after initial OS challenge.
Figure 5.
Addition of CD36Ab after OS binding to RPE is necessary and sufficient to increase the rate of OS internalization by RPE, independently of the OS binding receptor αvβ5. Differentiated monolayers of RPE-J cells were challenged for 2 h with FITC-OS in the presence of 50 μg/ml preimmune IgG or 50 μg/ml rat CD36 IgG. After 2 h, some samples were fixed to determine binding and internalization indices as shown in A. Striped bars, OS binding; filled bars, OS internalization; −, preimmune IgG control; +, CD36 IgG. (B) Identical samples as in A were washed with assay buffer 2 h after OS addition to remove unbound OS and further incubated in presence of either preimmune (−) or CD36 IgG (+). B shows the internalization indices of these samples determined 4 h after initial OS challenge (2 h after the removal of unbound OS). (C) We repeated the experiments shown in A and B but added αvβ5 inhibiting Ab P1F6 to all samples during the 2 h of OS challenge. P1F6 reduced OS binding at 2 h equally well in the presence of preimmune IgG, (−, dark striped bars) or of CD36 IgG (+, light striped bars). Internalization indices for both conditions were insignificant after 2 h (2 h, dark and light filled bars). Samples that were incubated with OS and P1F6 for 2 h were washed and incubated for another 2 h in the presence of preimmune (−) or CD36 IgG (+). Internalization indices that were determined after a total time of incubation of 4 h demonstrated that CD36 IgG accelerated internalization of OS prebound in the presence of P1F6 (4 h; −, preimmune IgG, +, CD36 IgG added after removal of unbound OS at 2 h). Note that the y axis scale in C is different from that in A and B. Values in A–C represent average OS indices ± SD (n = 3). (B and C). Statistical evaluation using Student's t test determined P < 0.001 for all changes compared with the appropriate control condition that were discussed as significant in the text. Note that all cells regardless of treatment completed internalization of prebound OS within 6 h after initial OS challenge (data not shown). Between the removal of unbound OS at 2 h and the termination of the experiment, the total amount of OS detected in each sample remained constant (data not shown). Thus, surface-bound OS did not dissociate from RPE cells and RPE cells did not degrade phagocytosed OS between 2 and 6 h after initial OS challenge.
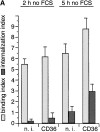
Figure 6.
CD36 Abs activate the OS internalization function of RPE in the absence of soluble factors. Differentiated monolayers of RPE-J cells were rinsed twice with warm serum free medium before challenge with OS resuspended in serum free medium in the presence of 50 μg/ml either of nonimmune IgG (n. i.), CD36 IgG (CD36), or oxLDL for 2 h or 5 h. Bars represent averages ±SD of three independent experiments. (A) Bars in A show binding (light bars) and internalization (dark bars) of OS in the absence of serum. Binding occurred in the absence of serum regardless of the stimulation of CD36. CD36 Abs increased internalization compared with nonimmune IgG (P < 0.02). (B) Cells received OS in the absence of serum for 2 h, at which unbound OS were removed. Cells were further incubated with or without 3% FCS (labeled: >2 h FCS, + or −) and in the presence of either preimmune or CD36 IgG (labeled: >2 h CD36 IgG, − or +). B shows the internalization indices of these samples determined 5 h after initial OS challenge (3 h after the removal of unbound OS). Remarkably, CD36 Abs were sufficient to accelerate internalization of prebound OS in the absence of serum (P < 0.001). (C) OxLDL (but not LDL, data not shown) had no effect on OS binding but increased OS internalization in the presence or absence of serum, as indicated in the Figure, during 5 h of coincubation with OS (P < 0.005). Thus, this multivalent CD36 ligand mimicked the effect observed with intact Ab.
Figure 6.
CD36 Abs activate the OS internalization function of RPE in the absence of soluble factors. Differentiated monolayers of RPE-J cells were rinsed twice with warm serum free medium before challenge with OS resuspended in serum free medium in the presence of 50 μg/ml either of nonimmune IgG (n. i.), CD36 IgG (CD36), or oxLDL for 2 h or 5 h. Bars represent averages ±SD of three independent experiments. (A) Bars in A show binding (light bars) and internalization (dark bars) of OS in the absence of serum. Binding occurred in the absence of serum regardless of the stimulation of CD36. CD36 Abs increased internalization compared with nonimmune IgG (P < 0.02). (B) Cells received OS in the absence of serum for 2 h, at which unbound OS were removed. Cells were further incubated with or without 3% FCS (labeled: >2 h FCS, + or −) and in the presence of either preimmune or CD36 IgG (labeled: >2 h CD36 IgG, − or +). B shows the internalization indices of these samples determined 5 h after initial OS challenge (3 h after the removal of unbound OS). Remarkably, CD36 Abs were sufficient to accelerate internalization of prebound OS in the absence of serum (P < 0.001). (C) OxLDL (but not LDL, data not shown) had no effect on OS binding but increased OS internalization in the presence or absence of serum, as indicated in the Figure, during 5 h of coincubation with OS (P < 0.005). Thus, this multivalent CD36 ligand mimicked the effect observed with intact Ab.
Figure 6.
CD36 Abs activate the OS internalization function of RPE in the absence of soluble factors. Differentiated monolayers of RPE-J cells were rinsed twice with warm serum free medium before challenge with OS resuspended in serum free medium in the presence of 50 μg/ml either of nonimmune IgG (n. i.), CD36 IgG (CD36), or oxLDL for 2 h or 5 h. Bars represent averages ±SD of three independent experiments. (A) Bars in A show binding (light bars) and internalization (dark bars) of OS in the absence of serum. Binding occurred in the absence of serum regardless of the stimulation of CD36. CD36 Abs increased internalization compared with nonimmune IgG (P < 0.02). (B) Cells received OS in the absence of serum for 2 h, at which unbound OS were removed. Cells were further incubated with or without 3% FCS (labeled: >2 h FCS, + or −) and in the presence of either preimmune or CD36 IgG (labeled: >2 h CD36 IgG, − or +). B shows the internalization indices of these samples determined 5 h after initial OS challenge (3 h after the removal of unbound OS). Remarkably, CD36 Abs were sufficient to accelerate internalization of prebound OS in the absence of serum (P < 0.001). (C) OxLDL (but not LDL, data not shown) had no effect on OS binding but increased OS internalization in the presence or absence of serum, as indicated in the Figure, during 5 h of coincubation with OS (P < 0.005). Thus, this multivalent CD36 ligand mimicked the effect observed with intact Ab.
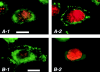
Figure 7.
CD36 Ab binding of CD36 at the RPE basal surface stimulates internalization of OS bound to αvβ5 at the apical surface. RPE-J cells adherent on coverslips coated with nonimmune Ab (A) or CD36 Ab (B) for 6 h were stained with murine CD36 Ab. 2 x-y sections of each field are shown, corresponding to the basal surface plane (A-1, B-1) and to a plane 6 μm above the basal surface (A-2, B-2), representing a subapical-lateral plane of an RPE-J cell. Cover glass staining caused by coating Ab was subtracted from basal fields as background. Total height of cells seeded on coated overslips was ∼8–10 μm (data not shown). While most RPE cells were in contact with neighboring cells 6 h after seeding, cells exhibited membrane protrusions (A2, B1) and had not established a polarized phenotype. Green signals shows CD36 labeling while red nuclei stain serves as reference. Scale bars: 10 μm. In RPE-J cells on control Ab, CD36 localized to both basal and subapical plasma membrane (A-1, A-2). In contrast, in cells on CD36 Ab, CD36 was prominent at basal attachment sites (B-1) and mostly absent from the subapical plane (B-2) suggesting that the CD36 Ab trapped CD36 at the glass surface. (C) During 2 h of OS challenge, RPE-J cells on control or on CD36 Ab bound FITC-OS normally, independent of the presence (+) or absence (−) of FCS. αvβ5 inhibitory Ab P1F6 at 50 μg/ml interfered with OS binding (+, αvβ5 Ab) Shown are average OS indices ± SD (n = 3). (D) During 4 h of OS challenge, cells on nonimmune Ab internalized OS in the presence (+) but not the absence (−) of serum. In contrast, cells on CD36 Ab internalized increased numbers of OS in the absence of serum (−) (averages ± SD, n = 4, P < 0.005). Soluble CD36 Ab at 100 μg/ml that reduced internalization by control cells (averages ± SD, n = 3, P < 0.01) had no effect on internalization by cells whose CD36 was trapped at the basal surface.
Figure 7.
CD36 Ab binding of CD36 at the RPE basal surface stimulates internalization of OS bound to αvβ5 at the apical surface. RPE-J cells adherent on coverslips coated with nonimmune Ab (A) or CD36 Ab (B) for 6 h were stained with murine CD36 Ab. 2 x-y sections of each field are shown, corresponding to the basal surface plane (A-1, B-1) and to a plane 6 μm above the basal surface (A-2, B-2), representing a subapical-lateral plane of an RPE-J cell. Cover glass staining caused by coating Ab was subtracted from basal fields as background. Total height of cells seeded on coated overslips was ∼8–10 μm (data not shown). While most RPE cells were in contact with neighboring cells 6 h after seeding, cells exhibited membrane protrusions (A2, B1) and had not established a polarized phenotype. Green signals shows CD36 labeling while red nuclei stain serves as reference. Scale bars: 10 μm. In RPE-J cells on control Ab, CD36 localized to both basal and subapical plasma membrane (A-1, A-2). In contrast, in cells on CD36 Ab, CD36 was prominent at basal attachment sites (B-1) and mostly absent from the subapical plane (B-2) suggesting that the CD36 Ab trapped CD36 at the glass surface. (C) During 2 h of OS challenge, RPE-J cells on control or on CD36 Ab bound FITC-OS normally, independent of the presence (+) or absence (−) of FCS. αvβ5 inhibitory Ab P1F6 at 50 μg/ml interfered with OS binding (+, αvβ5 Ab) Shown are average OS indices ± SD (n = 3). (D) During 4 h of OS challenge, cells on nonimmune Ab internalized OS in the presence (+) but not the absence (−) of serum. In contrast, cells on CD36 Ab internalized increased numbers of OS in the absence of serum (−) (averages ± SD, n = 4, P < 0.005). Soluble CD36 Ab at 100 μg/ml that reduced internalization by control cells (averages ± SD, n = 3, P < 0.01) had no effect on internalization by cells whose CD36 was trapped at the basal surface.
Similar articles
- Integrin alphavbeta5 is not required for the phagocytosis of photoreceptor outer segments by cultured retinal pigment epithelial cells.
Hall MO, Abrams TA, Burgess BL. Hall MO, et al. Exp Eye Res. 2003 Sep;77(3):281-6. doi: 10.1016/s0014-4835(03)00158-1. Exp Eye Res. 2003. PMID: 12907160 - CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium.
Ryeom SW, Sparrow JR, Silverstein RL. Ryeom SW, et al. J Cell Sci. 1996 Feb;109 ( Pt 2):387-95. doi: 10.1242/jcs.109.2.387. J Cell Sci. 1996. PMID: 8838662 - Alphavbeta5 integrin receptors at the apical surface of the RPE: one receptor, two functions.
Nandrot EF, Chang Y, Finnemann SC. Nandrot EF, et al. Adv Exp Med Biol. 2008;613:369-75. doi: 10.1007/978-0-387-74904-4_43. Adv Exp Med Biol. 2008. PMID: 18188966 Free PMC article. Review. No abstract available. - Morphogenesis of the retinal pigment epithelium: toward understanding retinal degenerative diseases.
Marmorstein AD, Finnemann SC, Bonilha VL, Rodriguez-Boulan E. Marmorstein AD, et al. Ann N Y Acad Sci. 1998 Oct 23;857:1-12. doi: 10.1111/j.1749-6632.1998.tb10102.x. Ann N Y Acad Sci. 1998. PMID: 9917828 Review.
Cited by
- Using flow cytometry to compare the dynamics of photoreceptor outer segment phagocytosis in iPS-derived RPE cells.
Westenskow PD, Moreno SK, Krohne TU, Kurihara T, Zhu S, Zhang ZN, Zhao T, Xu Y, Ding S, Friedlander M. Westenskow PD, et al. Invest Ophthalmol Vis Sci. 2012 Sep 14;53(10):6282-90. doi: 10.1167/iovs.12-9721. Invest Ophthalmol Vis Sci. 2012. PMID: 22871841 Free PMC article. - Macrophage ADAM17 deficiency augments CD36-dependent apoptotic cell uptake and the linked anti-inflammatory phenotype.
Driscoll WS, Vaisar T, Tang J, Wilson CL, Raines EW. Driscoll WS, et al. Circ Res. 2013 Jun 21;113(1):52-61. doi: 10.1161/CIRCRESAHA.112.300683. Epub 2013 Apr 12. Circ Res. 2013. PMID: 23584255 Free PMC article. - Deficits in Monocyte Function in Age Related Macular Degeneration: A Novel Systemic Change Associated With the Disease.
Gu BJ, Huang X, Avula PK, Caruso E, Drysdale C, Vessey KA, Ou A, Fowler C, Liu TH, Lin Y, Horton A, Masters CL, Wiley JS, Guymer RH, Fletcher EL. Gu BJ, et al. Front Med (Lausanne). 2021 Mar 17;8:634177. doi: 10.3389/fmed.2021.634177. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33816525 Free PMC article. - Progressive morphological and functional defects in retinas from alpha1 integrin-null mice.
Peng YW, Zallocchi M, Meehan DT, Delimont D, Chang B, Hawes N, Wang W, Cosgrove D. Peng YW, et al. Invest Ophthalmol Vis Sci. 2008 Oct;49(10):4647-54. doi: 10.1167/iovs.08-2011. Epub 2008 Jul 9. Invest Ophthalmol Vis Sci. 2008. PMID: 18614805 Free PMC article. - Apoptotic cell recognition receptors and scavenger receptors.
Penberthy KK, Ravichandran KS. Penberthy KK, et al. Immunol Rev. 2016 Jan;269(1):44-59. doi: 10.1111/imr.12376. Immunol Rev. 2016. PMID: 26683144 Free PMC article. Review.
References
- Edwards, R.B., and R.B. Szamier. 1977. Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science. 197:1001–1003. - PubMed
- LaVail, M.M. 1976. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 194:1071–1074. - PubMed
- Boyle, D., L.F. Tien, N.G. Cooper, V. Shepherd, and B.J. McLaughlin. 1991. A mannose receptor is involved in retinal phagocytosis. Invest. Ophthalmol. Vis Sci. 32:1464–1470. - PubMed
- D'Cruz, P.M., D. Yasumura, J. Weir, M.T. Matthes, H. Abderrahim, M.M. LaVail, and D. Vollrath. 2000. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 9:645–651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources