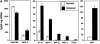Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment - PubMed (original) (raw)
Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment
M J Janatpour et al. J Exp Med. 2001.
Abstract
Monocytes recruited from the blood are key contributors to the nature of an immune response. While monocyte recruitment in a subset of immunopathologies has been well studied and largely attributed to the chemokine monocyte chemoattractant protein (MCP)-1, mechanisms mediating such recruitment to other sites of inflammation remain elusive. Here, we showed that localized inflammation resulted in an increased binding of monocytes to perifollicular high endothelial venules (HEVs) of lymph nodes draining a local inflammatory site. Quantitative PCR analyses revealed the upregulation of many chemokines in the inflamed lymph node, including MCP-1 and MIG. HEVs did not express detectable levels of MCP-1; however, a subset of HEVs in inflamed lymph nodes in wild-type (but not tumor necrosis factor [TNF] null mice) expressed MIG and this subset of HEVs preferentially supported monocyte binding. Expression of CXCR3, the receptor for MIG, was detected on a small subset of peripheral blood monocytes and on a significant percentage of recruited monocytes. Most importantly, in both ex vivo and in vivo assays, neutralizing anti-MIG antibodies blocked monocyte binding to inflamed lymph node HEVs. Together, these results suggest that the lymph node microenvironment can dictate the nature of molecules expressed on HEV subsets in a TNF-dependent fashion and that inflammation-induced MIG expression by HEVs can mediate monocyte recruitment.
Figures
Figure 1.
Real-time quantitative PCR (TaqMan™) analyses of chemokine expression in lymph nodes. Total RNA was isolated from pools of lymph nodes draining inflamed footpads (black bars) and pools of lymph nodes draining the contralateral footpads that were not inflamed (Normal, white bars). Real-time quantitative PCR was performed on 50 ng of reverse-transcribed cDNA using primers that specifically recognize a panel of chemokines. (A) mRNA levels for the subset of chemokines known to chemoattract monocytes in vitro and for MIG are shown. Data is expressed as fg per 50 ng cDNA. With the exception of RANTES, there was a relative increase in all the chemokines known to chemoattract monocytes, upon inflammation. P value < 0.005. Additionally, there was a greater than ninefold increase in the mRNA levels of MIG. (B) mRNA levels for a subset of chemokines in normal (not inflamed) and inflamed lymph nodes from TNF null mice are shown.
Figure 1.
Real-time quantitative PCR (TaqMan™) analyses of chemokine expression in lymph nodes. Total RNA was isolated from pools of lymph nodes draining inflamed footpads (black bars) and pools of lymph nodes draining the contralateral footpads that were not inflamed (Normal, white bars). Real-time quantitative PCR was performed on 50 ng of reverse-transcribed cDNA using primers that specifically recognize a panel of chemokines. (A) mRNA levels for the subset of chemokines known to chemoattract monocytes in vitro and for MIG are shown. Data is expressed as fg per 50 ng cDNA. With the exception of RANTES, there was a relative increase in all the chemokines known to chemoattract monocytes, upon inflammation. P value < 0.005. Additionally, there was a greater than ninefold increase in the mRNA levels of MIG. (B) mRNA levels for a subset of chemokines in normal (not inflamed) and inflamed lymph nodes from TNF null mice are shown.
Figure 2.
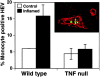
Recruitment of monocytes to lymph node HEVs is increased in wild-type mice, but not in TNF null mice, upon inflammation. 3 d after inducing inflammation, lymph nodes draining either inflamed footpads (black bars) or footpads that were not inflamed (white bars) were embedded in O.C.T., sectioned and subjected to the snapshot assay. Double-indirect immunohistochemistry was performed to identify all HEVs (red; see inset) and monocytes (green). The data is presented as percentage of total HEVs with at least one bound monocyte. Inducing inflammation in the footpads of wild-type mice results in nearly a threefold increase in monocyte recruitment to lymph node HEVs. TNF null mice have a reduced ability to recruit monocytes to HEVs upon inflammation, compared to wild-type mice. This experiment was repeated four times for wild-type mice and three times for TNF null mice. P value < 0.05.
Figure 3.
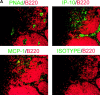
MIG expression on HEVs is correlated with increased monocyte-selective recruitment. (A) Serial sections of lymph nodes draining inflamed footpads were stained with antibodies against PNAd (top left panel, green) to identify HEVs, IP10 (top right panel, green), MCP-1 (bottom left panel, green), or an isotype control (bottom right panel, green). On all sections, an antibody against the B-cell marker B220 (red) identified the follicles and was used for orientation. MCP-1 and IP10 was not displayed on HEVs, but rather in macrophage-rich areas. (B) Serial sections (left and middle panels) were stained with either antibodies against PNAd (left panel, green) and B220 (left panel, red) or MIG (middle panel, green) and 6CKine (middle panel, red). MIG was expressed on a subset of 6CKine+ HEVs. White arrows pair vessels between left and middle panels; yellow arrow indicates a vessel that is absent in the section represented in the middle panel. The right panel depicts vessels that were stained in vitro for 6CKine (red) and MIG (green). The outline depicts the B cell follicle border. Arrows depict vessels in which expression is completely colocalized (white) or partially colocalized (yellow). (C) Serial sections of lymph nodes draining inflamed footpads were stained for PNAd (left panels, green) and CD11b (red) or MIG (right panels, green) and CD11b (red). MIG+ HEVs showed a higher association with monocytes than MIG− HEVs (arrows indicate MIG− vessels). (D) Serial sections were stained with antibodies against PNAd and MIG. The number of PNAd+ and MIG+ vessels was then counted across 10 lymph nodes. Data is presented as the percentage of total HEVs that were MIG+. Lymph nodes that were not inflamed (white bar) had <2% MIG+ HEVs. Upon inflammation (black bar), the number of MIG+ HEVs increased to ∼12%. This experiment was repeated twice. (E) Immunohistochemistry was performed as shown in C. All PNAd+ (total HEVs) and MIG+ vessels were counted and scored for whether a monocyte was bound. Data is presented as percentage of HEV+ for at least one bound monocyte. Upon inflammation (black bars) this increased from ∼6% in lymph nodes that were not inflamed (white bar) to ∼20%. The percentage of the subpopulation of total inflamed HEVs that were MIG+ and had a bound monocyte was >60%; whereas, the percentage of the MIG− HEV subpopulation that had a bound monocyte was only 13%. This experiment was repeated twice.
Figure 3.
MIG expression on HEVs is correlated with increased monocyte-selective recruitment. (A) Serial sections of lymph nodes draining inflamed footpads were stained with antibodies against PNAd (top left panel, green) to identify HEVs, IP10 (top right panel, green), MCP-1 (bottom left panel, green), or an isotype control (bottom right panel, green). On all sections, an antibody against the B-cell marker B220 (red) identified the follicles and was used for orientation. MCP-1 and IP10 was not displayed on HEVs, but rather in macrophage-rich areas. (B) Serial sections (left and middle panels) were stained with either antibodies against PNAd (left panel, green) and B220 (left panel, red) or MIG (middle panel, green) and 6CKine (middle panel, red). MIG was expressed on a subset of 6CKine+ HEVs. White arrows pair vessels between left and middle panels; yellow arrow indicates a vessel that is absent in the section represented in the middle panel. The right panel depicts vessels that were stained in vitro for 6CKine (red) and MIG (green). The outline depicts the B cell follicle border. Arrows depict vessels in which expression is completely colocalized (white) or partially colocalized (yellow). (C) Serial sections of lymph nodes draining inflamed footpads were stained for PNAd (left panels, green) and CD11b (red) or MIG (right panels, green) and CD11b (red). MIG+ HEVs showed a higher association with monocytes than MIG− HEVs (arrows indicate MIG− vessels). (D) Serial sections were stained with antibodies against PNAd and MIG. The number of PNAd+ and MIG+ vessels was then counted across 10 lymph nodes. Data is presented as the percentage of total HEVs that were MIG+. Lymph nodes that were not inflamed (white bar) had <2% MIG+ HEVs. Upon inflammation (black bar), the number of MIG+ HEVs increased to ∼12%. This experiment was repeated twice. (E) Immunohistochemistry was performed as shown in C. All PNAd+ (total HEVs) and MIG+ vessels were counted and scored for whether a monocyte was bound. Data is presented as percentage of HEV+ for at least one bound monocyte. Upon inflammation (black bars) this increased from ∼6% in lymph nodes that were not inflamed (white bar) to ∼20%. The percentage of the subpopulation of total inflamed HEVs that were MIG+ and had a bound monocyte was >60%; whereas, the percentage of the MIG− HEV subpopulation that had a bound monocyte was only 13%. This experiment was repeated twice.
Figure 3.
MIG expression on HEVs is correlated with increased monocyte-selective recruitment. (A) Serial sections of lymph nodes draining inflamed footpads were stained with antibodies against PNAd (top left panel, green) to identify HEVs, IP10 (top right panel, green), MCP-1 (bottom left panel, green), or an isotype control (bottom right panel, green). On all sections, an antibody against the B-cell marker B220 (red) identified the follicles and was used for orientation. MCP-1 and IP10 was not displayed on HEVs, but rather in macrophage-rich areas. (B) Serial sections (left and middle panels) were stained with either antibodies against PNAd (left panel, green) and B220 (left panel, red) or MIG (middle panel, green) and 6CKine (middle panel, red). MIG was expressed on a subset of 6CKine+ HEVs. White arrows pair vessels between left and middle panels; yellow arrow indicates a vessel that is absent in the section represented in the middle panel. The right panel depicts vessels that were stained in vitro for 6CKine (red) and MIG (green). The outline depicts the B cell follicle border. Arrows depict vessels in which expression is completely colocalized (white) or partially colocalized (yellow). (C) Serial sections of lymph nodes draining inflamed footpads were stained for PNAd (left panels, green) and CD11b (red) or MIG (right panels, green) and CD11b (red). MIG+ HEVs showed a higher association with monocytes than MIG− HEVs (arrows indicate MIG− vessels). (D) Serial sections were stained with antibodies against PNAd and MIG. The number of PNAd+ and MIG+ vessels was then counted across 10 lymph nodes. Data is presented as the percentage of total HEVs that were MIG+. Lymph nodes that were not inflamed (white bar) had <2% MIG+ HEVs. Upon inflammation (black bar), the number of MIG+ HEVs increased to ∼12%. This experiment was repeated twice. (E) Immunohistochemistry was performed as shown in C. All PNAd+ (total HEVs) and MIG+ vessels were counted and scored for whether a monocyte was bound. Data is presented as percentage of HEV+ for at least one bound monocyte. Upon inflammation (black bars) this increased from ∼6% in lymph nodes that were not inflamed (white bar) to ∼20%. The percentage of the subpopulation of total inflamed HEVs that were MIG+ and had a bound monocyte was >60%; whereas, the percentage of the MIG− HEV subpopulation that had a bound monocyte was only 13%. This experiment was repeated twice.
Figure 3.
MIG expression on HEVs is correlated with increased monocyte-selective recruitment. (A) Serial sections of lymph nodes draining inflamed footpads were stained with antibodies against PNAd (top left panel, green) to identify HEVs, IP10 (top right panel, green), MCP-1 (bottom left panel, green), or an isotype control (bottom right panel, green). On all sections, an antibody against the B-cell marker B220 (red) identified the follicles and was used for orientation. MCP-1 and IP10 was not displayed on HEVs, but rather in macrophage-rich areas. (B) Serial sections (left and middle panels) were stained with either antibodies against PNAd (left panel, green) and B220 (left panel, red) or MIG (middle panel, green) and 6CKine (middle panel, red). MIG was expressed on a subset of 6CKine+ HEVs. White arrows pair vessels between left and middle panels; yellow arrow indicates a vessel that is absent in the section represented in the middle panel. The right panel depicts vessels that were stained in vitro for 6CKine (red) and MIG (green). The outline depicts the B cell follicle border. Arrows depict vessels in which expression is completely colocalized (white) or partially colocalized (yellow). (C) Serial sections of lymph nodes draining inflamed footpads were stained for PNAd (left panels, green) and CD11b (red) or MIG (right panels, green) and CD11b (red). MIG+ HEVs showed a higher association with monocytes than MIG− HEVs (arrows indicate MIG− vessels). (D) Serial sections were stained with antibodies against PNAd and MIG. The number of PNAd+ and MIG+ vessels was then counted across 10 lymph nodes. Data is presented as the percentage of total HEVs that were MIG+. Lymph nodes that were not inflamed (white bar) had <2% MIG+ HEVs. Upon inflammation (black bar), the number of MIG+ HEVs increased to ∼12%. This experiment was repeated twice. (E) Immunohistochemistry was performed as shown in C. All PNAd+ (total HEVs) and MIG+ vessels were counted and scored for whether a monocyte was bound. Data is presented as percentage of HEV+ for at least one bound monocyte. Upon inflammation (black bars) this increased from ∼6% in lymph nodes that were not inflamed (white bar) to ∼20%. The percentage of the subpopulation of total inflamed HEVs that were MIG+ and had a bound monocyte was >60%; whereas, the percentage of the MIG− HEV subpopulation that had a bound monocyte was only 13%. This experiment was repeated twice.
Figure 3.
MIG expression on HEVs is correlated with increased monocyte-selective recruitment. (A) Serial sections of lymph nodes draining inflamed footpads were stained with antibodies against PNAd (top left panel, green) to identify HEVs, IP10 (top right panel, green), MCP-1 (bottom left panel, green), or an isotype control (bottom right panel, green). On all sections, an antibody against the B-cell marker B220 (red) identified the follicles and was used for orientation. MCP-1 and IP10 was not displayed on HEVs, but rather in macrophage-rich areas. (B) Serial sections (left and middle panels) were stained with either antibodies against PNAd (left panel, green) and B220 (left panel, red) or MIG (middle panel, green) and 6CKine (middle panel, red). MIG was expressed on a subset of 6CKine+ HEVs. White arrows pair vessels between left and middle panels; yellow arrow indicates a vessel that is absent in the section represented in the middle panel. The right panel depicts vessels that were stained in vitro for 6CKine (red) and MIG (green). The outline depicts the B cell follicle border. Arrows depict vessels in which expression is completely colocalized (white) or partially colocalized (yellow). (C) Serial sections of lymph nodes draining inflamed footpads were stained for PNAd (left panels, green) and CD11b (red) or MIG (right panels, green) and CD11b (red). MIG+ HEVs showed a higher association with monocytes than MIG− HEVs (arrows indicate MIG− vessels). (D) Serial sections were stained with antibodies against PNAd and MIG. The number of PNAd+ and MIG+ vessels was then counted across 10 lymph nodes. Data is presented as the percentage of total HEVs that were MIG+. Lymph nodes that were not inflamed (white bar) had <2% MIG+ HEVs. Upon inflammation (black bar), the number of MIG+ HEVs increased to ∼12%. This experiment was repeated twice. (E) Immunohistochemistry was performed as shown in C. All PNAd+ (total HEVs) and MIG+ vessels were counted and scored for whether a monocyte was bound. Data is presented as percentage of HEV+ for at least one bound monocyte. Upon inflammation (black bars) this increased from ∼6% in lymph nodes that were not inflamed (white bar) to ∼20%. The percentage of the subpopulation of total inflamed HEVs that were MIG+ and had a bound monocyte was >60%; whereas, the percentage of the MIG− HEV subpopulation that had a bound monocyte was only 13%. This experiment was repeated twice.
Figure 4.
A function-perturbing antibody to MIG blocks monocyte binding to inflamed HEVs in vivo. To test whether MIG plays a role in monocyte recruitment to inflamed lymph nodes in vivo, inflammation was induced in both forepaws of 12 mice. The mice were divided into two groups, each receiving either a function-perturbing antibody to MIG or a control antibody intraperitoneally. In one experiment (Exp. 2), 12 mice that were not inflamed (white bars) also received the antibody treatment. Draining lymph nodes were isolated and sectioned. (A) To confirm that the antibodies reached and bound the HEVs, sections were stained with a FITC-conjugated secondary antibody to visualize the intraperitoneally injected antibody. Sections were costained with an antibody against B220. The neutralizing antibody to MIG localized to the HEV (right, green). (B) The in vivo snapshot assay was performed to determine the percentage of HEVs with a bound monocyte in the presence of either a MIG neutralizing antibody (αMIG) or the control (Cont Ab). 15–20% of inflamed HEVs had a bound monocyte in the presence of the control antibody, similar to what was observed in the absence of antibody (compare No Ab to Cont Ab, black bars). In the presence of a MIG neutralizing antibody this percentage was reduced to levels equivalent to uninflamed controls (white bars).
Figure 4.
A function-perturbing antibody to MIG blocks monocyte binding to inflamed HEVs in vivo. To test whether MIG plays a role in monocyte recruitment to inflamed lymph nodes in vivo, inflammation was induced in both forepaws of 12 mice. The mice were divided into two groups, each receiving either a function-perturbing antibody to MIG or a control antibody intraperitoneally. In one experiment (Exp. 2), 12 mice that were not inflamed (white bars) also received the antibody treatment. Draining lymph nodes were isolated and sectioned. (A) To confirm that the antibodies reached and bound the HEVs, sections were stained with a FITC-conjugated secondary antibody to visualize the intraperitoneally injected antibody. Sections were costained with an antibody against B220. The neutralizing antibody to MIG localized to the HEV (right, green). (B) The in vivo snapshot assay was performed to determine the percentage of HEVs with a bound monocyte in the presence of either a MIG neutralizing antibody (αMIG) or the control (Cont Ab). 15–20% of inflamed HEVs had a bound monocyte in the presence of the control antibody, similar to what was observed in the absence of antibody (compare No Ab to Cont Ab, black bars). In the presence of a MIG neutralizing antibody this percentage was reduced to levels equivalent to uninflamed controls (white bars).
Figure 5.
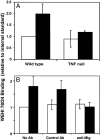
MIG may play a direct role in monocyte-selective recruitment in inflammation. (A) Frozen lymph node sections were incubated with cells under nonstatic, cold conditions, and the ability of the HEVs to bind WEHI 78/24 was quantitated. Data is expressed as WEHI binding relative to an internal standard. Lymph nodes from inflamed (black bars) wild-type mice had a twofold increase in WEHI 78/24 binding to HEVs when compared to mice that were not inflamed (white bar; P value < 0.01). Furthermore, monocyte binding to inflamed HEVs from TNF null mice was similar to that observed in uninflamed wild-type mice, analogous to what was observed in vivo. This experiment was repeated three times. (B) The ex vivo assay described previously was performed on both inflamed lymph nodes (black bars) or lymph nodes that were not inflamed (white bars) in the presence of either a MIG neutralizing antibody (anti-MIG), a control (Control Ab), or no antibody at all (No Ab). In the presence of the control antibody, there was approximately a twofold increase in monocyte binding to inflamed HEVs compared to uninflamed HEVs (P value < 0.05). This was similar to that observed in the absence of antibody. In the presence of a MIG-neutralizing antibody, however, the degree of monocyte binding to inflamed HEVs was reduced to background levels. This experiment was repeated three times.
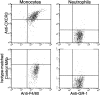
Figure 6.
Newly recruited monocytes express CXCR3. Balb/C mice were given a single intraperitoneal injection of thyoglycollate. 2 d later, peritoneal cells were harvested and stained for CXCR3 or an isotype control (CyC), CD11b (FITC), F4/80 (PE), and GR1 (APC). Gates were set on newly recruited monocytes (CD11b+) and neutrophils (GR1+), after selection based on size and side-scatter. A significant percentage of monocytes recruited to the peritoneum expressed CXCR3 (compared to isotype control) whereas the neutrophil population did not.
Comment in
- New mechanisms and pathways for monocyte recruitment.
Muller WA. Muller WA. J Exp Med. 2001 Nov 5;194(9):F47-51. doi: 10.1084/jem.194.9.f47. J Exp Med. 2001. PMID: 11696603 Free PMC article. Review. No abstract available.
Similar articles
- Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues.
Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Palframan RT, et al. J Exp Med. 2001 Nov 5;194(9):1361-73. doi: 10.1084/jem.194.9.1361. J Exp Med. 2001. PMID: 11696600 Free PMC article. - Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules.
Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, Narumi S, Morikawa S, Ezaki T, Lu B, Gerard C, Ishikawa S, Matsushima K. Yoneyama H, et al. Int Immunol. 2004 Jul;16(7):915-28. doi: 10.1093/intimm/dxh093. Epub 2004 May 24. Int Immunol. 2004. PMID: 15159375 - New mechanisms and pathways for monocyte recruitment.
Muller WA. Muller WA. J Exp Med. 2001 Nov 5;194(9):F47-51. doi: 10.1084/jem.194.9.f47. J Exp Med. 2001. PMID: 11696603 Free PMC article. Review. No abstract available. - High endothelial venules (HEVs) in immunity, inflammation and cancer.
Blanchard L, Girard JP. Blanchard L, et al. Angiogenesis. 2021 Nov;24(4):719-753. doi: 10.1007/s10456-021-09792-8. Epub 2021 May 6. Angiogenesis. 2021. PMID: 33956259 Free PMC article. Review.
Cited by
- In Sickness and in Health: The Immunological Roles of the Lymphatic System.
Johnson LA. Johnson LA. Int J Mol Sci. 2021 Apr 24;22(9):4458. doi: 10.3390/ijms22094458. Int J Mol Sci. 2021. PMID: 33923289 Free PMC article. Review. - Chemokine requirements for B cell entry to lymph nodes and Peyer's patches.
Okada T, Ngo VN, Ekland EH, Förster R, Lipp M, Littman DR, Cyster JG. Okada T, et al. J Exp Med. 2002 Jul 1;196(1):65-75. doi: 10.1084/jem.20020201. J Exp Med. 2002. PMID: 12093871 Free PMC article. - An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4.
Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P. Lasagni L, et al. J Exp Med. 2003 Jun 2;197(11):1537-49. doi: 10.1084/jem.20021897. J Exp Med. 2003. PMID: 12782716 Free PMC article. - Interferons induce CXCR3-cognate chemokine production by human metastatic melanoma.
Dengel LT, Norrod AG, Gregory BL, Clancy-Thompson E, Burdick MD, Strieter RM, Slingluff CL Jr, Mullins DW. Dengel LT, et al. J Immunother. 2010 Nov-Dec;33(9):965-74. doi: 10.1097/CJI.0b013e3181fb045d. J Immunother. 2010. PMID: 20948440 Free PMC article. - Rhinovirus-induces progression of lung disease in a mouse model of COPD via IL-33/ST2 signaling axis.
Gimenes JA Jr, Srivastava V, ReddyVari H, Kotnala S, Mishra R, Farazuddin M, Li W, Sajjan US. Gimenes JA Jr, et al. Clin Sci (Lond). 2019 Apr 29;133(8):983-996. doi: 10.1042/CS20181088. Print 2019 Apr 30. Clin Sci (Lond). 2019. PMID: 30952808 Free PMC article.
References
- Randolph, G.J., K. Inaba, D.F. Robbiani, R.M. Steinman, and W.A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 11:753–761. - PubMed
- Navab, M., S.Y. Hama, T.B. Nguyen, and A.M. Fogelman. 1994. Monocyte adhesion and transmigration in atherosclerosis. Coron. Artery Dis. 5:198–204. - PubMed
- Schmitz, G., A.S. Herr, and G. Rothe. 1998. T-lymphocytes and monocytes in atherogenesis. Herz. 23:168–177. - PubMed
- Reape, T.J., and P.H. Groot. 1999. Chemokines and atherosclerosis. Atherosclerosis. 147:213–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous