Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis - PubMed (original) (raw)
Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis
W He et al. J Neurosci. 2001.
Abstract
During CNS development, cell migrations play an important role, adding to the cellular complexity of different regions. Earlier studies have shown a robust migration of cells from basal forebrain into the overlying dorsal forebrain during the embryonic period. These immigrant cells include GABAergic neurons that populate the cerebral cortex and hippocampus. In this study we have examined the fate of other basal forebrain cells that migrate into the dorsal forebrain, identifying basal cells using an antibody that recognizes both early (dlx1/2) and late (dlx 5/6) members of the dlx homeobox gene family. We found that a subpopulation of cortical and hippocampal oligodendrocytes are also ventral-derived. We traced the origin of these cells to basal multipotent stem cells capable of generating both GABAergic neurons and oligodendrocytes. A clonal analysis showed that basal forebrain stem cells produce significantly more GABAergic neurons than dorsal forebrain stem cells from the same embryonic age. Moreover, stem cell clones from basal forebrain are significantly more likely to contain both GABAergic neurons and oligodendrocytes than those from dorsal. This indicates that forebrain stem cells are regionally specified. Whereas dlx expression was not detected within basal stem cells growing in culture, these cells produced dlx-positive products that are capable of migration. These data indicate that the developing cerebral cortex incorporates both neuronal and glial products of basal forebrain and suggest that these immigrant cells arise from a common progenitor, a dlx-negative basal forebrain stem cell.
Figures
Fig. 1.
Dlx expression in E14 mouse brain.A, Dlx is predominantly expressed in cells in the LGE at E14. Streams of dlx-positive cells are visible leading from basal forebrain into the cerebral cortex (small arrows). A higher magnification of the boxed area in_A_ is shown in B. _Arrows_indicate examples of dlx-positive nuclei entering the intermediate zone of the cortex. CTX, Cerebral cortex; LGE, lateral ganglionic eminence. Scale: A, 1 cm = 182 μm; B, 1 cm = 60 μm.
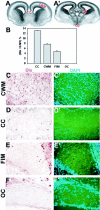
Fig. 2.
Dlx expression in developing white matter regions of E18 mouse brain. Low-power micrograph of coronal sections from E18 mouse brain illustrating the major white matter tracts in anterior (A) and posterior (A′) forebrain. CC, Corpus callosum;CWM, subcortical white matter; _FIM,_fimbria; OC, optic chiasm. B, The number of dlx-positive cells in sections of embryonic white matter tracts as a percentage of total cells (examples of staining are shown in_C–F_′). There was a significant difference among the different areas of white matter (ANOVA; p < 0.01)._C–F_′, Examples of different areas of white matter in E18 brain sections stained for dlx (brown) (C–F) and counterstained with DAPI (blue) (_C_′–_F_′). Note that in dlx-positive nuclei, DAPI staining is not visible, hence the total cell number is calculated by adding the number of DAPI-positive and dlx-positive cells. Dlx-positive cells were found in the cortical white matter, corpus callosum, and fimbria, but not in the optic chiasm. Numbers of dlx-positive cells in different forebrain white matter areas are compared in B. Scale: 1 cm = 91 μm.
Fig. 3.
NG2-positive cells expressing dlx in E18 mouse corpus callosum. E18 mouse brain sections were stained with anti-dlx and with NG2, a marker expressed by oligodendrocyte progenitor cells. A, B, Low-power light micrograph of the same field of a section of E18 corpus callosum double-labeled for dlx in_brown_ (A) and for NG2 with a Cy3-labeled secondary antibody (B). C, D, High magnification of the boxed areas in_A_ and B, respectively.Arrows indicate a cell double-labeled for dlx and NG2. Scale bars: A, 50 μm; C, 25 μm.
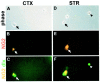
Fig. 4.
NG2-positive cells acutely isolated from E18 mouse forebrain express dlx. E18 cortical and striatal tissue was enzymatically dissociated with papain to single cells, which were fixed acutely and stained for NG2 and dlx. A–C, Cortical cells; D–F, striatal cells. A, D, Phase images of cells acutely isolated from E18 cortex and striatum.B, E, NG2 staining of cells in A and_D_. Arrows indicate NG2-positive cells (red). C, F, Double staining of cells in_A_ and D for NG2 (yellow) and dlx (green).Arrows indicate cells double-labeled for NG2 and dlx.Arrowhead in F indicates a cell that is dlx-positive but NG2-negative. Scale: 1 cm = 46.7 μm.
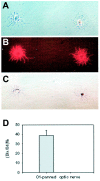
Fig. 5.
Dlx expression in O1-immunopanned cortical oligodendrocytes. Postmitotic oligodendrocytes (O1-positive) were immunoselected from P5 rat cerebral cortex. The cells were fixed acutely and stained with O4 antibody to confirm their oligodendrocyte identity and dlx. A, Phase image of two O1-immunoselected P5 cortical cells. B, O4 staining of cells in A. C, The oligodendrocyte on the_left_ is dlx-negative, whereas the one on the_right_ is dlx-positive, with typical nuclear staining.D, The percentage of dlx-positive cells in O1-immunopanned P5 cortical cells was 38%. In contrast, no dlx-positive cells were found in oligodendrocytes isolated from the P5 optic nerve. Scale: 1 cm = 48 μm.
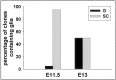
Fig. 6.
Oligodendroglia generating progenitor cells derived from E11.5 and E13 basal forebrain. Progenitor cells were isolated from basal forebrain and plated at clonal density and followed in culture for 5–12 d before staining for NG2 or O4 and β-tubulin III. Note that progenitor cells giving purely glial progeny were very rare at young ages and increased with development from E11.5 to E13. Hence, at early ages most oligodendrocytes arise from stem cells that also make neurons. G, Pure glial clones;SC, stem cell clones.
Fig. 7.
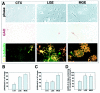
A clonal analysis of stem cells from dorsal and basal E13 forebrain reveals differences in their production of GABAergic neurons and oligodendrocytes. A, Progenitor cells were isolated from three forebrain regions at E13: cortex (CTX), lateral ganglionic eminence (LGE), and medial ganglionic eminence (MGE). The cells were cultured for 12 d and stained for GAD, O4, and β-tubulin III. Stem cell (SC) clones that contained both GABAergic neurons and oligodendrocytes were found in all three regions, as illustrated in A. β-tubulin III-positive neurons are shown in green, O4-positive oligodendrocytes in yellow, and GAD-positive neurons in_brown_. Scale: 1 cm = 58 μm.B, The number of GAD-positive neurons expressed as a percentage of total neurons produced by progenitor cells from cortex, LGE, and MGE. There is a significant difference between the percentage of GAD-positive neurons made by cortical progenitors and the percentage made by LGE or MGE progenitor cells (ANOVA analysis;p < 0.001). tub, β-tubulin III.C, The number of GAD-positive neurons expressed as a percentage of total neurons produced within stem cell clones was calculated. MGE stem cells generated significantly more GAD-positive neurons than LGE or cortical stem cells (ANOVA analysis;p < 0.05). D, The percentage of stem cell clones containing both GABAergic neurons and oligodendrocytes was calculated for the three forebrain regions. LGE and MGE produced significantly more stem cell clones containing both these cell types than did cortex (ANOVA analysis; p < 0.05).GABA-N, GABAergic neuron; oligo, oligodendrocyte.
Fig. 8.
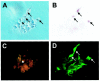
Dlx expression in a developing stem cell clone. Single cells from E13 forebrain were dissociated and plated at clonal density. Growing clones were mapped and followed every day for 5 d. Stem cell clones were stained for NG2 to identify glial progeny and β-tubulin III to identify neurons and dlx. A, Phase micrograph of a developing stem cell clone at 5 d in vitro. B, After staining for dlx, three cells are positive, as indicated by arrows. _C,_Membranous NG2 staining of the same clone (small arrow_points to an NG2-positive cell seen in phase in A and in_red fluorescence in C). _D,_β-tubulin III staining of the same clone (_arrows_indicate that the three dlx-positive cells seen in B are also β-tubulin III-positive). Scale: 1 cm = 50 μm.
Similar articles
- Differential modulation of BMP signaling promotes the elaboration of cerebral cortical GABAergic neurons or oligodendrocytes from a common sonic hedgehog-responsive ventral forebrain progenitor species.
Yung SY, Gokhan S, Jurcsak J, Molero AE, Abrajano JJ, Mehler MF. Yung SY, et al. Proc Natl Acad Sci U S A. 2002 Dec 10;99(25):16273-8. doi: 10.1073/pnas.232586699. Epub 2002 Dec 2. Proc Natl Acad Sci U S A. 2002. PMID: 12461181 Free PMC article. - Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain.
Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. Petryniak MA, et al. Neuron. 2007 Aug 2;55(3):417-33. doi: 10.1016/j.neuron.2007.06.036. Neuron. 2007. PMID: 17678855 Free PMC article. - GABAergic Interneuron Differentiation in the Basal Forebrain Is Mediated through Direct Regulation of Glutamic Acid Decarboxylase Isoforms by Dlx Homeobox Transcription Factors.
Le TN, Zhou QP, Cobos I, Zhang S, Zagozewski J, Japoni S, Vriend J, Parkinson T, Du G, Rubenstein JL, Eisenstat DD. Le TN, et al. J Neurosci. 2017 Sep 6;37(36):8816-8829. doi: 10.1523/JNEUROSCI.2125-16.2017. Epub 2017 Aug 8. J Neurosci. 2017. PMID: 28821666 Free PMC article. - DLX genes and proteins in mammalian forebrain development.
Rubenstein JL, Nord AS, Ekker M. Rubenstein JL, et al. Development. 2024 Jun 1;151(11):dev202684. doi: 10.1242/dev.202684. Epub 2024 May 31. Development. 2024. PMID: 38819455 Review. - The distribution of Dlx1-2 and glutamic acid decarboxylase in the embryonic and adult hypothalamus reveals three differentiated LHA subdivisions in rodents.
Barbier M, Croizier S, Alvarez-Bolado G, Risold PY. Barbier M, et al. J Chem Neuroanat. 2022 Apr;121:102089. doi: 10.1016/j.jchemneu.2022.102089. Epub 2022 Mar 10. J Chem Neuroanat. 2022. PMID: 35283254 Review.
Cited by
- Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain.
Ventura RE, Goldman JE. Ventura RE, et al. J Neurosci. 2007 Apr 18;27(16):4297-302. doi: 10.1523/JNEUROSCI.0399-07.2007. J Neurosci. 2007. PMID: 17442813 Free PMC article. - Generation of Cre-transgenic mice using Dlx1/Dlx2 enhancers and their characterization in GABAergic interneurons.
Potter GB, Petryniak MA, Shevchenko E, McKinsey GL, Ekker M, Rubenstein JL. Potter GB, et al. Mol Cell Neurosci. 2009 Feb;40(2):167-86. doi: 10.1016/j.mcn.2008.10.003. Epub 2008 Nov 1. Mol Cell Neurosci. 2009. PMID: 19026749 Free PMC article. - Human fetal radial glia cells generate oligodendrocytes in vitro.
Mo Z, Zecevic N. Mo Z, et al. Glia. 2009 Apr 1;57(5):490-8. doi: 10.1002/glia.20775. Glia. 2009. PMID: 18814269 Free PMC article. - Oct4-induced reprogramming is required for adult brain neural stem cell differentiation into midbrain dopaminergic neurons.
Deleidi M, Cooper O, Hargus G, Levy A, Isacson O. Deleidi M, et al. PLoS One. 2011;6(5):e19926. doi: 10.1371/journal.pone.0019926. Epub 2011 May 31. PLoS One. 2011. PMID: 21655272 Free PMC article. - Synapses on NG2-expressing progenitors in the brain: multiple functions?
Gallo V, Mangin JM, Kukley M, Dietrich D. Gallo V, et al. J Physiol. 2008 Aug 15;586(16):3767-81. doi: 10.1113/jphysiol.2008.158436. Epub 2008 Jul 17. J Physiol. 2008. PMID: 18635642 Free PMC article. Review.
References
- Akiyama Y, Honmou O, Kato T, Uede T, Hashi K, Kocsis JD. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167:27–39. - PubMed
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
- Barres BA, Koroshetz WJ, Swartz KJ, Chun LL, Corey DP. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990;4:507–524. - PubMed
- Betarbet R, Zigova T, Bakay RA, Luskin MB. Dopaminergic and GABAergic interneurons of the olfactory bulb are derived from the neonatal subventricular zone. Int J Dev Neurosci. 1996;14:921–930. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials