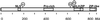Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein - PubMed (original) (raw)
Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein
T Kawaguchi et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Recently we purified and identified a previously uncharacterized transcription factor from rat liver binding to the carbohydrate responsive element of the L-type pyruvate kinase (L-PK) gene. This factor was named carbohydrate responsive element binding protein (ChREBP). ChREBP, essential for L-PK gene transcription, is activated by high glucose and inhibited by cAMP. Here, we demonstrated that (i) nuclear localization signal and basic helix-loop-helix/leucine-zipper domains of ChREBP were essential for the transcription, and (ii) these domains were the targets of regulation by cAMP and glucose. Among three cAMP-dependent protein kinase phosphorylation sites, Ser(196) and Thr(666) were the target sites. Phosphorylation of the former resulted in inactivation of nuclear import, and that of the latter resulted in loss of the DNA-binding activity and L-PK transcription. On the other hand, glucose activated the nuclear import by dephosphorylation of Ser(196) in the cytoplasm and also stimulated the DNA-binding activity by dephosphorylation of Thr(666) in the nucleus. These results thus reveal mechanisms for regulation of ChREBP and the L-PK transcription by excess carbohydrate and cAMP.
Figures
Figure 1
Schematic representation of domain structure of mouse ChREBP. The locations of ChREBP, NLS, PRO, bHLH/ZIP, and ZIP-like domains are indicated. The locations of three putative phosphorylation sites of cAMP-dependent protein kinase (PKA) are indicated as P1, P2, and P3.
Figure 2
Effects of domain deletion of ChREBP on transcription activity of the L-PK gene. Rat primary cultured hepatocytes were transfected with the WT ChREBP or a series of domain deletion mutants. A pGL3 basic plasmid (simian virus 40 promoter driving firefly luciferase gene), carrying the promoter region between positions −206 and −7 of the L-PK gene, and pRL-TK (thymidine kinase promoter driving the Renilla luciferase gene) were also transfected into each cell as a reporter gene and an internal control, respectively. After transfection, cells were incubated under 5.5 mM (□) or 27.5 mM (■) glucose for 12 h. Relative luciferase activity was calculated as described in Experimental Procedures and are expressed as mean ± SEM (n = 5). *, P < 0.05 compared with that of WT ChREBP.
Figure 3
Effects of H-89 and cantharidic acid on transcription activity of L-PK gene in ChREBP-overexpressed hepatocytes. Rat primary cultured hepatocytes were transfected with the WT ChREBP. H-89, cantharidic acid, or dimethyl sulfoxide vehicle was added, and the cells were incubated in 5.5 mM (□) or 27.5 mM (■) glucose for 12 h. Relative luciferase activity was calculated as described in Experimental Procedures and is expressed as mean ± SEM (n = 5). *, P < 0.05 compared with that of WT ChREBP.
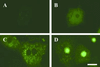
Figure 4
Representative images of subcellular localization of GFP-fused ChREBP under low or high glucose. (A) Nontransfected hepatocytes; (B) Empty vector-transfected hepatocytes; (C) GFP-fused WT ChREBP transfected hepatocyte in low (5.5 mM) glucose; (D) GFP-fused WT ChREBP transfected hepatocyte in low (27.5 mM) glucose. (Bar = 10 μm.)
Figure 5
(A) Effect of NLS domain deletion, H-89, and cantharidic acid on nuclear translocation of ChREBP. Rat primary cultured hepatocytes were transfected with GFP-fused WT ChREBP or NLS domain deletion mutants. H-89, cantharidic acid, or dimethyl sulfoxide vehicle was added, and the cells were incubated under 5.5 mM (□) or 27.5 mM (■) glucose for 12 h. For each condition, 100 transfected hepatocytes from four independent experiments were scored in a blinded fashion as to whether the GFP-fused ChREBP was predominantly nuclear or cytoplasmic as described in Experimental Procedures. The values are expressed as mean ± SEM. *, P < 0.05 compared with that of WT ChREBP. (B) Putative PKA phosphorylation sites. The phosphorylation sites, Ser or Thr, are underlined. Ala or Asp introduced in the place of Ser or Thr, respectively, by site-directed mutagenesis are underlined. (C) Effects of mutation of Ser196 on subcellular localization of ChREBP. Rat primary cultured hepatocytes were transfected with GFP-fused WT ChREBP, S196A, or S196D, and the cells were incubated in 5.5 mM (□) or 27.5 mM (■) glucose for 12 h. For each condition, 100 transfected hepatocytes from four independent experiments were scored in a blinded fashion as to whether the GFP-fused ChREBP was predominantly nuclear or cytoplasmic as described in Experimental Procedures. The values are expressed as mean ± SEM. *, P < 0.05 compared with that of WT ChREBP.
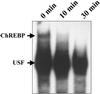
Figure 6
Effect of db-cAMP on the DNA-binding activity of ChREBP in vivo. Rats were fed high carbohydrate diets after 48 h of starvation, and db-cAMP (60 mg/kg) was injected intraperitoneally. The rats were killed 10 and 30 min after the injection, and the DNA-binding activity of ChREBP was examined by using a gel shift assay. USF, upstream stimulatory factor.
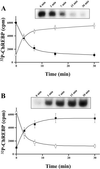
Figure 7
(A) Phosphorylation of ChREBP by PKA and effects of PKA on the DNA-binding activity of ChREBP in vitro. Recombinant C-terminal ChREBP was incubated with PKA in the presence of [γ-32P]ATP. At given time intervals, [32P]phosphate incorporation (□) and DNA-binding activity (■) were determined as described in Experimental Procedures. The values are expressed as mean ± SEM. (Insert) Representative image for effects of PKA on the DNA-binding activity of ChREBP using gel shift assay. (B) Dephosphorylation of ChREBP by PP2A and the effect of PP2A on the DNA-binding activity of ChREBP in vitro. Phosphorylated C-terminal ChREBP was incubated with PP2A. At given time intervals, remaining phosphorylated C-terminal ChREBP (□) and DNA-binding activity (■) were determined as described in Experimental Procedures. The values are expressed as mean ± SEM. (Insert) Representative image for effects of PP2A on the DNA-binding activity of ChREBP.
Figure 8
(A) The effect of mutation at Ser626 and Thr666 on the transcription activity of the L-PK gene. Rat primary cultured hepatocytes were transfected with WT ChREBP, S626A, S626D, T666A, or T666D. After transfection, the cells were incubated in 5.5 mM (□) or 27.5 mM (■) glucose for 12 h. Relative luciferase activity was calculated as described in Experimental Procedures and are expressed as mean ± SEM (n = 5). *, P < 0.05 compared with that of WT ChREBP. (B) The effects of db-cAMP on L-PK gene transcription in S626A- and T666A-overexpressed hepatocytes. To exclude the effect of db-cAMP on nuclear translocation of ChREBP, double mutants (S196A + S626A and S196A + T666A) were constructed. Rat primary cultured hepatocytes were transfected with WT ChREBP, S196A + S626A, or S196A + T666A. After transfection, cells were incubated under 5.5 mM (□) or 27.5 mM (■) glucose, and cAMP was added to both low and high glucose. Relative luciferase activity was calculated as described in Experimental Procedures and are expressed as mean ± SEM (n = 5). *, P < 0.05 compared with that of WT ChREBP.
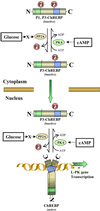
Figure 9
Schematic representation of possible regulatory mechanisms of ChREBP by glucose and cAMP in hepatocytes. Two PKA phosphorylation sites of ChREBP, Ser196 (P1) and Thr666 (P3), play crucial roles in high glucose-induced activation of ChREBP (P1 and P3 indicate the phosphorylated form at the sites). Glucose signaling may activate PP2A via a metabolite (X). In cytoplasm, X-activated PP2A dephosphorylates P1 site of the ChREBP, which results in stimulation of import of ChREBP into the nucleus. Once ChREBP is localized in the nucleus, glucose activates the inactive form of ChREBP (P3-ChREBP) by dephosphorylation of the P3 site catalyzed by PP2A. ChREBP, which is dephosphorylated at P1 and P3 sites, binds to ChRE of the L-PK gene and consequently activates transcription of the L-PK gene. See Discussion.
Comment in
- Glucose and cAMP: adversaries in the regulation of hepatic gene expression.
Towle HC. Towle HC. Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13476-8. doi: 10.1073/pnas.251530798. Proc Natl Acad Sci U S A. 2001. PMID: 11717416 Free PMC article. Review. No abstract available. - Molecular scaffolds for chemical wizardry: learning nature's rules for terpene cyclases.
Greenhagen B, Chappell J. Greenhagen B, et al. Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13479-81. doi: 10.1073/pnas.261562898. Proc Natl Acad Sci U S A. 2001. PMID: 11717417 Free PMC article. Review. No abstract available.
Similar articles
- Mechanism for fatty acid "sparing" effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase.
Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Kawaguchi T, et al. J Biol Chem. 2002 Feb 8;277(6):3829-35. doi: 10.1074/jbc.M107895200. Epub 2001 Nov 27. J Biol Chem. 2002. PMID: 11724780 - Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver.
Kabashima T, Kawaguchi T, Wadzinski BE, Uyeda K. Kabashima T, et al. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5107-12. doi: 10.1073/pnas.0730817100. Epub 2003 Apr 8. Proc Natl Acad Sci U S A. 2003. PMID: 12684532 Free PMC article. - Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes.
Stoeckman AK, Ma L, Towle HC. Stoeckman AK, et al. J Biol Chem. 2004 Apr 9;279(15):15662-9. doi: 10.1074/jbc.M311301200. Epub 2004 Jan 23. J Biol Chem. 2004. PMID: 14742444 - Glucose and cAMP: adversaries in the regulation of hepatic gene expression.
Towle HC. Towle HC. Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13476-8. doi: 10.1073/pnas.251530798. Proc Natl Acad Sci U S A. 2001. PMID: 11717416 Free PMC article. Review. No abstract available. - Carbohydrate responsive element-binding protein (ChREBP): a key regulator of glucose metabolism and fat storage.
Uyeda K, Yamashita H, Kawaguchi T. Uyeda K, et al. Biochem Pharmacol. 2002 Jun 15;63(12):2075-80. doi: 10.1016/s0006-2952(02)01012-2. Biochem Pharmacol. 2002. PMID: 12110366 Review.
Cited by
- A novel N-terminal domain may dictate the glucose response of Mondo proteins.
McFerrin LG, Atchley WR. McFerrin LG, et al. PLoS One. 2012;7(4):e34803. doi: 10.1371/journal.pone.0034803. Epub 2012 Apr 10. PLoS One. 2012. PMID: 22506051 Free PMC article. - MondoA coordinately regulates skeletal myocyte lipid homeostasis and insulin signaling.
Ahn B, Soundarapandian MM, Sessions H, Peddibhotla S, Roth GP, Li JL, Sugarman E, Koo A, Malany S, Wang M, Yea K, Brooks J, Leone TC, Han X, Vega RB, Kelly DP. Ahn B, et al. J Clin Invest. 2016 Sep 1;126(9):3567-79. doi: 10.1172/JCI87382. Epub 2016 Aug 8. J Clin Invest. 2016. PMID: 27500491 Free PMC article. - A combined transcriptomics and lipidomics analysis of subcutaneous, epididymal and mesenteric adipose tissue reveals marked functional differences.
Caesar R, Manieri M, Kelder T, Boekschoten M, Evelo C, Müller M, Kooistra T, Cinti S, Kleemann R, Drevon CA. Caesar R, et al. PLoS One. 2010 Jul 12;5(7):e11525. doi: 10.1371/journal.pone.0011525. PLoS One. 2010. PMID: 20634946 Free PMC article. - Epigenetic-Transcriptional Regulation of Fatty Acid Metabolism and Its Alterations in Leukaemia.
Maher M, Diesch J, Casquero R, Buschbeck M. Maher M, et al. Front Genet. 2018 Sep 24;9:405. doi: 10.3389/fgene.2018.00405. eCollection 2018. Front Genet. 2018. PMID: 30319689 Free PMC article. Review. - Viperin regulates cellular lipid metabolism during human cytomegalovirus infection.
Seo JY, Cresswell P. Seo JY, et al. PLoS Pathog. 2013;9(8):e1003497. doi: 10.1371/journal.ppat.1003497. Epub 2013 Aug 1. PLoS Pathog. 2013. PMID: 23935494 Free PMC article.
References
- Kahn A. Biochimie. 1997;79:113–118. - PubMed
- Shih H M, Liu Z, Towle H C. J Biol Chem. 1995;270:21991–21997. - PubMed
- Vaulont S, Puzenat N, Levrat F, Cognet M, Kahn A, Raymondjean M J. Mol Biol. 1989;209:205–219. - PubMed
- Puzenat N, Vaulont S, Kahn A, Raymondjean M. Biochem Biophys Res Commun. 1992;189:1119–1128. - PubMed
- Koo S H, Towle H C. J Biol Chem. 2000;275:5200–5207. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases