PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine - PubMed (original) (raw)
PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine
T Okazaki et al. Proc Natl Acad Sci U S A. 2001.
Abstract
PD-1 is an immunoreceptor that belongs to the immunoglobulin (Ig) superfamily and contains two tyrosine residues in the cytoplasmic region. Studies on PD-1-deficient mice have shown that PD-1 plays critical roles in establishment and/or maintenance of peripheral tolerance, but the mode of action is totally unknown. To study the molecular mechanism for negative regulation of lymphocytes through the PD-1 receptor, we generated chimeric molecules composed of the IgG Fc receptor type IIB (Fc gamma RIIB) extracellular region and the PD-1 cytoplasmic region and expressed them in a B lymphoma cell line, IIA1.6. Coligation of the cytoplasmic region of PD-1 with the B cell receptor (BCR) in IIA1.6 transformants inhibited BCR-mediated growth retardation, Ca(2+) mobilization, and tyrosine phosphorylation of effector molecules, including Ig beta, Syk, phospholipase C-gamma 2 (PLC gamma 2), and ERK1/2, whereas phosphorylation of Lyn and Dok was not affected. Mutagenesis studies indicated that these inhibitory effects do not require the N-terminal tyrosine in the immunoreceptor tyrosine-based inhibitory motif-like sequence, but do require the other tyrosine residue in the C-terminal tail. This tyrosine was phosphorylated and recruited src homology 2-domain-containing tyrosine phosphatase 2 (SHP-2) on coligation of PD-1 with BCR. These results show that PD-1 can inhibit BCR signaling by recruiting SHP-2 to its phosphotyrosine and dephosphorylating key signal transducers of BCR signaling.
Figures
Figure 1
Strategies to dissect inhibitory mechanisms of PD-1. (A) Fc chimera and PD-1 constructs. The cytoplasmic regions of PD-1, its mutants, and KIR were fused with the extracellular region of FcγRIIB. A flag tag was added to each Fc chimera. (B) Cell surface expression of Fc chimeras and PD-1. Sorted Fc chimera and PD-1 transformants were stained with anti-FcγRIIB and anti-PD-1 mAb, respectively. GFP, green fluorescence protein. (C) Schematic representation of stimulation strategy. Incubation with intact anti-mouse IgG Ab results in coligation of Fc chimera and BCR (Right), whereas a F(ab′)2 fragment of anti-mouse IgG Ab results in BCR-only ligation (Left).
Figure 2
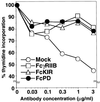
FcPD coligation with BCR inhibited BCR-mediated growth retardation in IIA1.6 cells. Mock, FcγRIIB, FcKIR, and FcPD transformants were stimulated with indicated concentrations of anti-mouse IgG Abs, and cell growth was determined by [3H]thymidine incorporation. Relative growth was calculated by dividing thymidine incorporation of stimulated cells by that of unstimulated cells. Each percentage is the mean of triplicate wells.
Figure 3
Inhibition of BCR-mediated Ca2+ mobilization by coligation with FcPD and PD-1. Cytosolic calcium concentrations of Fc-chimera transformants were measured with a KAF 110 spectrophotometer. Each transformant was stimulated by coligation of Fc chimera and BCR or ligation of BCR only. (A) FcPD inhibited BCR-mediated Ca2+ mobilization as efficiently as FcγRIIB and FcKIR. (B) FcPDF1 inhibited BCR-mediated Ca2+ mobilization, whereas FcPDF2, FcPDF1F2, and FcPDTM could not. (C) FcPD inhibited BCR-mediated Ca2+ mobilization in the presence of EGTA. (D) Inhibitory effect of full-length PD-1.
Figure 4
Recruitment and phosphorylation of SHP-2 to tyrosine-phosphorylated FcPD. (A) IIA1.6 cells expressing FcPD, FcγRIIB, and FcKIR were stimulated, and cell lysates were immunoprecipitated (IP) by anti-flag mAb and analyzed for interaction with SHP-2 by Western blotting. (B) FcPD-expressing IIA1.6 cells were stimulated with intact Abs or F(ab′)2 fragments of anti-mouse IgG for the indicated intervals. Tyrosine phosphorylation of FcPD, association between FcPD and SHP-2, and tyrosine phosphorylation of SHP-2 were determined as described in Materials and Methods. (C) Tyrosine phosphorylation of tyrosine mutants of FcPD and association between tyrosine mutants of FcPD and SHP-2 were determined as above. Closed and open arrowheads indicate full-length and truncated FcPD chimeras, respectively.
Figure 5
Coligation of PD-1 with BCR inhibited BCR-mediated tyrosine phosphorylation of various molecules. (A) Mock and FcPD transformants were stimulated, and cell lysates were immunoprecipitated (IP) with anti-phosphotyrosine Ab (anti-pY) and examined for phosphotyrosine contents by Western blotting. (B–G) Mock, FcPD, FcPDF1F2, FcγRIIB, or FcKIR transformants were stimulated under indicated conditions. Cell lysates were immunoprecipitated with Abs against Lyn (B), Igβ (C), Syk (D), PLCγ2 (E), and Dok (G); resolved by SDS/PAGE; transferred to membrane; and probed (immunoblotted, IB) with the Abs indicated. The closed arrowhead indicates the tyrosine-phosphorylated Igβ in C. The kinase activity of Lyn on enolase was also measured (B). Cell lysates were probed with anti-pERK1/2 (F). pERK1/2 represents the p44/42 ERK1 and ERK2, which are phosphorylated at Thr-202 and Tyr-204 and thus are activated. Closed and open arrowheads indicate ERK1 and ERK2, respectively, in F.
Similar articles
- Specificity of the SH2 domains of SHP-1 in the interaction with the immunoreceptor tyrosine-based inhibitory motif-bearing receptor gp49B.
Wang LL, Blasioli J, Plas DR, Thomas ML, Yokoyama WM. Wang LL, et al. J Immunol. 1999 Feb 1;162(3):1318-23. J Immunol. 1999. PMID: 9973385 - Regulation of B cell signal transduction by SH2-containing protein-tyrosine phosphatases.
Siminovitch KA, Neel BG. Siminovitch KA, et al. Semin Immunol. 1998 Aug;10(4):329-47. doi: 10.1006/smim.1998.0125. Semin Immunol. 1998. PMID: 9695189 Review. - A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail.
Poole AW, Jones ML. Poole AW, et al. Cell Signal. 2005 Nov;17(11):1323-32. doi: 10.1016/j.cellsig.2005.05.016. Cell Signal. 2005. PMID: 16084691 Review.
Cited by
- Association Between the Immune Checkpoint Inhibitor Durvalumab and Myasthenia Gravis: A Comprehensive Review.
Bector G, Trehan S, Toofantabrizi M, Singh G, Jain A, Arora N, Shrestha S, Panjwani GAR, Jain P, Kalra E. Bector G, et al. Cureus. 2024 Sep 3;16(9):e68542. doi: 10.7759/cureus.68542. eCollection 2024 Sep. Cureus. 2024. PMID: 39364500 Free PMC article. Review. - Immune checkpoint pathways in glioblastoma: a diverse and evolving landscape.
Inocencio JF, Mitrasinovic S, Asad M, Parney IF, Zang X, Himes BT. Inocencio JF, et al. Front Immunol. 2024 Sep 13;15:1424396. doi: 10.3389/fimmu.2024.1424396. eCollection 2024. Front Immunol. 2024. PMID: 39346924 Free PMC article. Review. - FBXO38 is dispensable for PD-1 regulation.
Dibus N, Salyova E, Kolarova K, Abdirov A, Pagano M, Stepanek O, Cermak L. Dibus N, et al. EMBO Rep. 2024 Oct;25(10):4206-4225. doi: 10.1038/s44319-024-00220-8. Epub 2024 Sep 12. EMBO Rep. 2024. PMID: 39266770 Free PMC article. - Exploring the Role of PD-1 in the Autoimmune Response: Insights into Its Implication in Systemic Lupus Erythematosus.
Sagrero-Fabela N, Chávez-Mireles R, Salazar-Camarena DC, Palafox-Sánchez CA. Sagrero-Fabela N, et al. Int J Mol Sci. 2024 Jul 15;25(14):7726. doi: 10.3390/ijms25147726. Int J Mol Sci. 2024. PMID: 39062968 Free PMC article. Review. - The pathogenic T42A mutation in SHP2 rewires the interaction specificity of its N-terminal regulatory domain.
van Vlimmeren AE, Voleti R, Chartier CA, Jiang Z, Karandur D, Humphries PA, Lo WL, Shah NH. van Vlimmeren AE, et al. Proc Natl Acad Sci U S A. 2024 Jul 23;121(30):e2407159121. doi: 10.1073/pnas.2407159121. Epub 2024 Jul 16. Proc Natl Acad Sci U S A. 2024. PMID: 39012820
References
- Shinohara T, Taniwaki M, Ishida Y, Kawaichi M, Honjo T. Genomics. 1994;23:704–706. - PubMed
- Bolland S, Ravetch J-V. Adv Immunol. 1999;72:149–177. - PubMed
- Unkeless J-C, Jin J. Curr Opin Immunol. 1997;9:338–343. - PubMed
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Int Immunol. 1996;8:765–772. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous