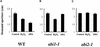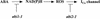Abscisic acid activation of plasma membrane Ca(2+) channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants - PubMed (original) (raw)
Abscisic acid activation of plasma membrane Ca(2+) channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants
Y Murata et al. Plant Cell. 2001 Nov.
Abstract
The hormone abscisic acid (ABA) regulates stress responses and developmental processes in plants. Calcium-permeable channels activated by reactive oxygen species (ROS) have been shown recently to function in the ABA signaling network in Arabidopsis guard cells. Here, we report that ABA activation of these I(Ca) Ca(2)+ channels requires the presence of NAD(P)H in the cytosol. The protein phosphatase 2C (PP2C) mutant abi1-1 disrupted ABA activation of I(Ca) channels. Moreover, in abi1-1, ABA did not induce ROS production. Consistent with these findings, in abi1-1, H(2)O(2) activation of I(Ca) channels and H(2)O(2)-induced stomatal closing were not disrupted, suggesting that abi1-1 impairs ABA signaling between ABA reception and ROS production. The abi2-1 mutation, which lies in a distinct PP2C gene, also disrupted ABA activation of I(Ca). However, in contrast to abi1-1, abi2-1 impaired both H(2)O(2) activation of I(Ca) and H(2)O(2)-induced stomatal closing. Furthermore, ABA elicited ROS production in abi2-1. These data suggest a model with the following sequence of events in early ABA signal transduction: ABA, abi1-1, NAD(P)H-dependent ROS production, abi2-1, I(Ca) Ca(2)+ channel activation followed by stomatal closing.
Figures
Figure 1.
ABA Activation of Ca2+-Permeable ICa Currents in Arabidopsis Guard Cell Protoplasts Requires Cytosolic NADPH. (A) and (B) ABA (50 μM) did not activate ICa calcium channels when the pipette solution did not include NADPH or NADH. (B) shows two overlapping traces from a guard cell before and after ABA application. (C) and (D) ABA (50 μM) activated ICa calcium currents when 5 mM NADPH was added to the pipette solution. (E) and (F) ABA activated ICa when 1 mM NADPH was added to the pipette solution. (A), (C), and (E) show average responses, and (B), (D), and (F) show responses in individual cells before and 5 min after ABA application. ABA was added ∼10 min after establishing whole-cell recordings, and whole-cell currents were measured before ABA application and in the same cells 5 min after extracellular ABA application in all recordings. The numbers of cells averaged are given in the text. Closed circles, before ABA addition to batch solution; open circles, 5 min after ABA addition to the same cells. Error bars represent
sem
.
Figure 2.
ABA Failed to Activate Ca2+ Channel Currents in abi1-1 and abi2-1 PP2C Mutant Guard Cells. (A) ABA (50 μM) did not activate ICa in abi1-1 guard cells with 5 mM NADPH included in the pipette solution (n = 5). (B) ABA (50 μM) did not activate ICa in abi2-1 guard cells with 5 mM NADPH added to the pipette solution (n = 5). Experiments were performed as described in Figure 1. Error bars represent
sem
.
Figure 3.
H2O2 Activates Ca2+-Permeable Currents in abi1-1 but Not abi2-1 Arabidopsis Guard Cell Protoplasts. (A) and (B) H2O2 (100 μM) activates ICa in abi1-1 guard cells (n = 6). (C) and (D) H2O2 (100 μM) did not activate ICa in abi2-1 guard cells (n = 5). (D) shows two overlapping traces. Experiments were performed as described in Figure 1 except that H2O2 was used instead of ABA. Error bars represent
sem
.
Figure 4.
Differential Effects of H2O2 on Stomatal Apertures of abi1-1 and abi2-1. (A) Both ABA (50 μM) and H2O2 (100 μM) induced stomatal closing in the wild type (Landsberg erecta ecotype). (B) ABA did not cause stomatal closing, but H2O2 elicited partial stomatal closing in abi1-1. (C) Neither ABA nor H2O2 elicited stomatal closing of abi2-1. Averages from n = three leaf epidermal experiments are shown (60 stomates per bar). Error bars represent
sem
.
Figure 5.
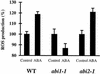
Differential Production of ROS in abi1-1 and abi2-1 Guard Cells Treated with ABA. ABA (50 μM) increased ROS production in the wild type (WT) (four experiments, n = 161 cells before ABA treatment, n = 123 cells after ABA treatment) and abi2-1 (nine experiments, n = 221 cells before ABA treatment, n = 207 cells after ABA treatment). ABA did not increase ROS production in abi1-1 (six experiments, n = 110 cells before ABA treatment, n = 150 cells after ABA treatment). Changes in ROS levels were analyzed by measuring H2DCF fluorescence levels in guard cells in response to ABA or solvent (0.1% ethanol) control applications. Error bars represent
sem
.
Figure 6.
Model Summarizing the Differential Disruption of ABA Activation of ICa Ca2+ Channels by the Two abi1-1 and abi2-1 PP2C Mutants. ABA activation of Ca2+ channels required cytosolic NAD(P)H, was inhibited by DPI, and was accompanied by ROS production.
Similar articles
- Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells.
Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI. Allen GJ, et al. Plant Cell. 1999 Sep;11(9):1785-98. doi: 10.1105/tpc.11.9.1785. Plant Cell. 1999. PMID: 10488243 Free PMC article. - The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production.
Munemasa S, Oda K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y, Murata Y. Munemasa S, et al. Plant Physiol. 2007 Mar;143(3):1398-407. doi: 10.1104/pp.106.091298. Epub 2007 Jan 12. Plant Physiol. 2007. PMID: 17220365 Free PMC article. - Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants.
Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Pei ZM, et al. Plant Cell. 1997 Mar;9(3):409-23. doi: 10.1105/tpc.9.3.409. Plant Cell. 1997. PMID: 9090884 Free PMC article. - Signal transduction and ion channels in guard cells.
MacRobbie EA. MacRobbie EA. Philos Trans R Soc Lond B Biol Sci. 1998 Sep 29;353(1374):1475-88. doi: 10.1098/rstb.1998.0303. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9800209 Free PMC article. Review. - Protein phosphatase 2C (PP2C) function in higher plants.
Rodriguez PL. Rodriguez PL. Plant Mol Biol. 1998 Dec;38(6):919-27. doi: 10.1023/a:1006054607850. Plant Mol Biol. 1998. PMID: 9869399 Review.
Cited by
- Transcriptome profiling of Arabidopsis slac1-3 mutant reveals compensatory alterations in gene expression underlying defective stomatal closure.
Wang Z, Ouyang Y, Ren H, Wang S, Xu D, Xin Y, Hussain J, Qi G. Wang Z, et al. Front Plant Sci. 2022 Sep 20;13:987606. doi: 10.3389/fpls.2022.987606. eCollection 2022. Front Plant Sci. 2022. PMID: 36204078 Free PMC article. - NAD - new roles in signalling and gene regulation in plants.
Hunt L, Lerner F, Ziegler M. Hunt L, et al. New Phytol. 2004 Jul;163(1):31-44. doi: 10.1111/j.1469-8137.2004.01087.x. New Phytol. 2004. PMID: 33873776 Review. - DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling.
He J, Duan Y, Hua D, Fan G, Wang L, Liu Y, Chen Z, Han L, Qu LJ, Gong Z. He J, et al. Plant Cell. 2012 May;24(5):1815-33. doi: 10.1105/tpc.112.098707. Epub 2012 May 31. Plant Cell. 2012. PMID: 22652060 Free PMC article. - Microbe Associated Molecular Pattern Signaling in Guard Cells.
Ye W, Murata Y. Ye W, et al. Front Plant Sci. 2016 May 4;7:583. doi: 10.3389/fpls.2016.00583. eCollection 2016. Front Plant Sci. 2016. PMID: 27200056 Free PMC article. Review. - The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor.
Laloi C, Mestres-Ortega D, Marco Y, Meyer Y, Reichheld JP. Laloi C, et al. Plant Physiol. 2004 Mar;134(3):1006-16. doi: 10.1104/pp.103.035782. Epub 2004 Feb 19. Plant Physiol. 2004. PMID: 14976236 Free PMC article.
References
- Allen, G.J., Kwak, J.M., Chu, S.P., Llopis, J., Tsien, R.Y., Harper, J.F., and Schroeder, J.I. (1999. b). Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 19 735–747. - PubMed
- Allen, G.J., Chu, S.P., Schumacher, K., Shimazaki, C.T., Vafeados, D., Kemper, A., Hawke, S.D., Tallman, G., Tsien, R.Y., Harper, J.F., Chory, J., and Schroeder, J.I. (2000). Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289 2338–2342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous