Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS - PubMed (original) (raw)
Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS
K P Fong et al. Infect Immun. 2001 Dec.
Abstract
The cell density-dependent control of gene expression is employed by many bacteria for regulating a variety of physiological functions, including the generation of bioluminescence, sporulation, formation of biofilms, and the expression of virulence factors. Although periodontal organisms do not appear to secrete acyl-homoserine lactone signals, several species, e.g., Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum, have recently been shown to secrete a signal related to the autoinducer II (AI-2) of the signal system 2 pathway in Vibrio harveyi. Here, we report that the periodontal pathogen Actinobacillus actinomycetemcomitans expresses a homolog of V. harveyi luxS and secretes an AI-2-like signal. Cell-free conditioned medium from A. actinomycetemcomitans or from a recombinant Escherichia coli strain (E. coli AIS) expressing A. actinomycetemcomitans luxS induced luminescence in V. harveyi BB170 >200-fold over controls. AI-2 levels peaked in mid-exponential-phase cultures of A. actinomycetemcomitans and were significantly reduced in late-log- and stationary-phase cultures. Incubation of early-log-phase A. actinomycetemcomitans cells with conditioned medium from A. actinomycetemcomitans or from E. coli AIS resulted in a threefold induction of leukotoxic activity and a concomitant increase in leukotoxin polypeptide. In contrast, no increase in leukotoxin expression occurred when cells were exposed to sterile medium or to conditioned broth from E. coli AIS(-), a recombinant strain in which luxS was insertionally inactivated. A. actinomycetemcomitans AI-2 also induced expression of afuA, encoding a periplasmic iron transport protein, approximately eightfold, suggesting that LuxS-dependent signaling may play a role in the regulation of iron acquisition by A. actinomycetemcomitans. Finally, A. actinomycetemcomitans AI-2 added in trans complemented a luxS knockout mutation in P. gingivalis by modulating the expression of the luxS-regulated genes uvrB and hasF in this organism. Together, these results suggest that LuxS-dependent signaling may modulate aspects of virulence and the uptake of iron by A. actinomycetemcomitans and induce responses in other periodontal organisms in mixed-species oral biofilm.
Figures
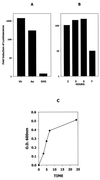
FIG. 1
AI-2 activity secreted by A. actinomycetemcomitans. (A) AI-2 activity in cell-free conditioned medium from an overnight V. harveyi BB170 culture (Vh) and from mid-exponential-phase A. actinomycetemcomitans (Aa) and E. coli DH5α (DH5) cultures was determined by monitoring the induction of V. harveyi luminescence as described in Materials and Methods. V. harveyi luminescence was determined after incubation of cells with the appropriate conditioned medium for 4 h. A negative control reaction (not shown) consisted of V. harveyi cells incubated with sterile medium. The increase in induction was calculated by dividing the light production of the experimental samples by that of the negative control. (B) AI-2 activity is maximal in mid-exponential-phase A. actinomycetemcomitans cultures. An overnight A. actinomycetemcomitans culture was diluted into fresh medium (1:20) and harvested after incubation at 37°C for 2, 3, 5, and 7 h. Cell-free conditioned medium was prepared from each sample and analyzed for AI-2 activity as described above. (C) Growth curve of A. actinomycetemcomitans JP2. O.D., optical density.
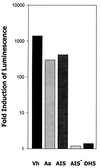
FIG. 2
AI-2 activity requires a functional luxS. AI-2 activity in cell-free conditioned medium from an overnight V. harveyi BB170 culture (Vh) and from mid-exponential-phase cultures of A. actinomycetemcomitans (Aa), E. coli DH5α (DH5), E. coli AIS, which expresses A. actinomycetemcomitans luxS in pGEMT-Easy, and E. coli AIS−, in which the plasmid-borne luxS was inactivated by insertion of a chloramphenicol resistance marker, was determined by monitoring the induction of V. harveyi luminescence as described in Materials and Methods. V. harveyi luminescence was determined after incubation of cells with the appropriate conditioned medium for 4 h. A negative control reaction consisted of V. harveyi cells incubated in sterile medium. The increase in induction was calculated by dividing the light production of the experimental samples by that of the negative control.
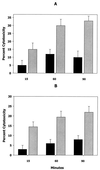
FIG. 3
Induction of leukotoxic activity by AI-2. The cytotoxicity of early-log-phase A. actinomycetemcomitans was determined after cells had been incubated for 15, 60, and 90 min in cell-free conditioned broth from mid-log-phase cultures (A, gray bars), sterile growth medium (A, black bars), conditioned medium from E. coli AIS (B, gray bars), or conditioned medium from E. coli AIS− (B, black bars). Error bars represent standard deviations; n = 3.
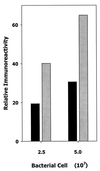
FIG. 4
Induction of leukotoxin protein by AI-2. Early-log-phase A. actinomycetemcomitans cells were incubated for 60 min in cell-free conditioned medium from E. coli AIS (gray bars) or AIS− (black bars) and spotted onto nitrocellulose. The filter was washed with PBS containing 1% bovine serum albumin and reacted with polyclonal antileukotoxin antibodies. Immunoreactivity was determined by measuring the relative intensity of spots on the developed filter using a Molecular Dynamics personal densitometer.
FIG. 5
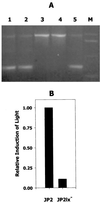
AI-2 stimulates the expression of afuA in A. actinomycetemcomitans. RT-PCR was carried out with primers that were specific for A. actinomycetemcomitans afuA (A) or cdtB (B) using 10 ng of total RNA from early-log-phase A. actinomycetemcomitans cells that had been exposed to conditioned medium from E. coli AIS (lane 1) or AIS− (lane 2). A 10-μl aliquot of the RT-PCR mixture was electrophoresed in 1% agarose. Lanes M, DNA size markers.
FIG. 6
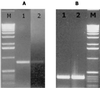
A. actinomycetemcomitans AI-2 complements a luxS knockout mutation in P. gingivalis. The wild-type P. gingivalis strain ATCC 33277 and its isogenic mutant (PLM1) in which luxS has been inactivated have been described (11). (A) RT-PCR using RNA isolated from P. gingivalis ATCC 33277 and PLM1 showed that inactivation of P. gingivalis luxS results in reduced expression of uvrB (lane 1) and increased expression of hasF (lane 2). (B) P. gingivalis PLM1 was subsequently incubated with cell-free conditioned broth from E. coli AIS or with sterile growth medium (sM) as described in Materials and Methods, and RT-PCRs were carried out using the _uvrB_- and _hasF_-specific primers used for panel A. Exposure to A. actinomycetemcomitans AI-2 induced the expression of uvrB (lane 1) and turned off hasF expression (lane 2). The fimA gene, which is not regulated by P. gingivalis luxS, was unaffected by exposure to A. actinomycetemcomitans AI-2 (lane 3).
FIG. 7
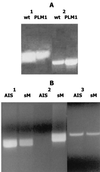
Isogenic _luxS_-deficient A. actinomycetemcomitans JP2. (A) Five kanamycin-resistant colonies of strain JP2 that had been transformed with pGEMT1.5K were analyzed by PCR using the primers Aa_luxS5 and Aa_luxS3 as described in Materials and Methods. Three clones (lanes 1, 2, and 5) contained the two amplification products predicted to result from single recombination of genomic luxS with pGEMT1.5K. The remaining clones (lanes 3 and 4) generated the single PCR product predicted to arise from the replacement of genomic luxS with the inactivated gene from pGEMT1.5K. Lane M, DNA size markers. (B) Conditioned culture medium from one of these clones (JP2lx−) was also analyzed and exhibited significantly reduced induction of luminescence from the V. harveyi BB170 reporter strain compared to conditioned medium from strain JP2. The induction of light by JP2 was arbitrarily assigned a value of 1.0.
References
- Bainton N J, Bycroft B W, Chhabra S, Stead P, Gledhill L, Hill P J, Rees C E D, Winson M K, Salmond G P C, Stewart G S A B, Williams P. A general role for the lux autoinducer in bacterial cell signaling: control of antibiotic biosynthesis in Erwinia. Gene. 1992;116:87–91. - PubMed
- Bassler B L. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol. 1999;2:582–587. - PubMed
- Bassler B L, Wright M, Showalter R E, Silverman M R. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. - PubMed
- Bassler B L, Wright M, Silverman M R. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994;13:273–286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases