Nog2p, a putative GTPase associated with pre-60S subunits and required for late 60S maturation steps - PubMed (original) (raw)
Nog2p, a putative GTPase associated with pre-60S subunits and required for late 60S maturation steps
C Saveanu et al. EMBO J. 2001.
Abstract
Eukaryotic ribosome maturation depends on a set of well ordered processing steps. Here we describe the functional characterization of yeast Nog2p (Ynr053cp), a highly conserved nuclear protein. Nog2p contains a putative GTP-binding site, which is essential in vivo. Kinetic and steady-state measurements of the levels of pre-rRNAs in Nog2p-depleted cells showed a defect in 5.8S and 25S maturation and a concomitant increase in the levels of both 27SB(S) and 7S(S) precursors. We found Nog2p physically associated with large pre-60S complexes highly enriched in the 27SB and 7S rRNA precursors. These complexes contained, besides a subset of ribosomal proteins, at least two additional factors, Nog1p, another putative GTP-binding protein, and Rlp24p (Ylr009wp), which belongs to the Rpl24e family of archaeal and eukaryotic ribosomal proteins. In the absence of Nog2p, the pre-60S ribosomal complexes left the nucleolus, but were retained in the nucleoplasm. These results suggest that transient, possibly GTP-dependent association of Nog2p with the pre-ribosomes might trigger late rRNA maturation steps in ribosomal large subunit biogenesis.
Figures
Fig. 1. Simplified maturation scheme for rRNA in yeast, adapted from Venema and Tollervey (1999). (A) Schematic representation of the large 35S primary pre-rRNA transcript. The relative position of the oligonucleotides used as probes in the study is also shown. (B) Processing of the 35S precursor to the mature rRNA species in yeast.
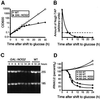
Fig. 2. Depletion of Nog2p (Ynr053cp) causes growth arrest and reduction of mature rRNA levels. (A) A conditionally lethal GAL::NOG2-TAP strain (LMA159) and a wild-type strain were grown at 30°C in YPGal and shifted to YPGlu medium. Optical densities were measured at various times of culture in glucose-containing medium. (B) Analysis of the Nog2p level in LMA159 cells at different times after the shift to glucose-containing medium. Proteins were extracted from the same number of cells and loaded on an 8% polyacrylamide gel. Immunoblotting with peroxidase–anti-peroxidase-soluble complexes revealed the protein A component of the tagged Nog2p. These levels were compared with the level measured for Nog2-TAP under the control of its own promoter (LMA78 strain) (horizontal dashed line). (C) Total RNAs were purified from either LMA159 or wild-type strains after growth in YPGlu for up to 40 h. RNAs were separated on a 1% agarose–formaldehyde gel and stained with ethidium bromide. (D) The levels of 25S and 18S mature rRNAs were normalized to the amount of U2 RNA; 100% corresponds to the galactose growth conditions.
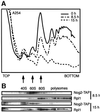
Fig. 3. Nog2p-depleted cells display a decrease in the abundance of free 60S ribosomal subunits. (A) Polysome profiles were analysed for the LMA159 strain by sedimentation through 10–50% sucrose gradients. Cells were either grown on YPGal (0 h) or shifted to glucose for 8.5 or 15 h. (B) Cellular proteins were extracted from various fractions of the sucrose gradient and analysed by immunoblotting using antibodies that revealed specifically either the Rpl1p (1:20 000 dilution) (Petitjean et al., 1995) or the Nog2-TAP proteins (peroxidase–anti-peroxidase 1:10 000 dilution).
Fig. 4. Nog2p depletion leads to reduced synthesis of 25S rRNA. Pulse–chase labelling with [methyl-3H]methionine was performed with the LMA159 strain (GAL::NOG2) grown in galactose medium (NOG2 induced) or shifted to glucose medium (NOG2 repressed) for 16 h. Cells were pulse-labelled for 2 min and then chased for the indicated times with an excess of cold methionine. Total labelled RNAs were purified, separated on a denaturing agarose gel and autoradiographed. The position of the intermediate and mature rRNAs is indicated.
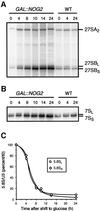
Fig. 5. Absence of Nog2p leads to an accumulation of the 27SBS and 7SS pre-rRNA. Total RNAs were extracted from the LMA159 strain after growth in YPGal or after shift to YPGlu for various times, as indicated. (A) Primer extensions were performed using primer CS10 (specific for the different 27S forms). The reaction products were resolved on a 5% acrylamide denaturing gel. (B) Northern blot analysis of 7S rRNA levels. The RNAs were separated on a 5% acrylamide denaturing gel, transferred to a nylon membrane and probed with oligonucleotide CS3, which hybridizes within the 7S region. (C) Quantification of the hybridization signals for the 5.8SS and 5.8SL mature rRNAs. The levels were normalized to the hybridization signals obtained with a U3 RNA-specific probe; 100% corresponds to the galactose-induced conditions.
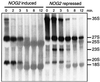
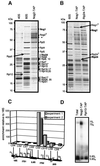
Fig. 6. Nog2p, Nog1p and Rlp24p are associated with similar pre-ribosomal complexes. (A) The protein composition of the affinity-purified Nog2p-associated complex (lane Nog2-TAP) was determined after electrophoresis on a gradient gel and Coomassie Blue staining. Proteins were identified using mass spectrometry and the pattern of protein bands was compared with the pattern of bands obtained with purified large (lane 60S) and small (lane 40S) ribosomal subunits. The tagged protein is marked Nog2T; a presumed Nog2p fragment is marked with *; a fragment of Rpl10p protein is marked Rpl10*. (B) Protein composition of the complexes obtained by tandem affinity purification using tagged versions of Nog1p (Nog1-TAP) and Rlp24p (Rlp24-TAP). Both Nog1p and Rlp24p were identified by mass spectrometry as components of the Rlp24p- and Nog1p-associated complexes, respectively. The tagged versions of the proteins are marked Nog1T and Rlp24T. For comparison, the proteins of the large ribosomal subunit were separated on the same gel (lane 60S). (C) Dot-blot quantitation of the different precursors of rRNAs in the Nog2p-associated complex. The RNAs were extracted from the purified complex, heat denatured, spotted on Hybond N+ membranes and probed with radiolabelled oligonucleotides specific for different rRNA precursors. The signal was quantified on a PhosphorImager system. The ratio of the signal obtained with the purified RNAs over the signal obtained with the total RNAs was calculated; these values were normalized by arbitrarily taking the ratio obtained with the 18S RNA probe (CS4) as a reference (equal to one on the figure). (D) Northern blot hybridization after polyacrylamide gel electrophoresis of total RNAs and RNAs purified in one step using TAP-tagged versions of Nog2p or ribosomal protein Rpl10-TAP used as a control. Radiolabelled oligonucleotide CS5, complementary to a region of the 5.8S mature rRNA, was used to probe the membrane. The positions of 5.8S and 7S RNAs are indicated. The enrichment for the 7S species in the Nog2p-associated RNAs can be observed by comparison with total RNAs or RNAs associated with Rpl10-TAP.
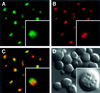
Fig. 7. Nog2p is located in the nucleolus and nucleoplasm. The LMA51-2 strain, containing the Nog2–GFP fusion under the control of its own promoter, was grown in SD-His medium. Cells were stained with Hoechst 33342 and photographed with filters specific for GFP fluorescence (A), Hoechst (B) or Nomarski (D). A merged image of (A) and (B) is shown in (C).
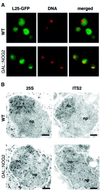
Fig. 8. Effect of Nog2p depletion on the localization of the pre-60S ribosomal particles. (A) Wild-type and GAL::NOG2 cells expressing Rpl25p–GFP were grown for 16 h in glucose-containing medium, stained with DAPI and photographed with filters for GFP fluorescence (L25–GFP) or DAPI (DNA); merge = images of the GFP and DAPI fluorescence merged. (B) In situ hybridization and electron microscopy were applied to wild-type and GAL::NOG2 cells grown for 16 h in glucose-containing medium using probes directed against the 25S rRNA sequence (25S) or with a probe complementary to ITS2 (ITS2).
Fig. 9. Conserved residues within the GTP-binding signature of Nog2p are essential for viability. (A) Sequences of predicted proteins from eukaryotes, archaea and bacteria that are similar to yeast Nog2p. Only the conserved G-regions are shown, with the position of the first residue marked. G2- and G5-like motifs (G2* and G5*) were detected in the Nog2p protein family by multiple alignments and secondary structure predictions (Materials and methods). Arrows point to the residues that have been substituted in the mutants (see text and C). Species origins of sequences are designated as follows, with SwissProt or TrEMBL accession numbers in parentheses: Sc, Saccharomyces cerevisiae (P53742); Hs, Homo sapiens (Q13823); Ce, Caenorhabditis elegans (Q9XXN4); Dm, Drosophila melanogaster (Q9V844); At, Arabidopsis thaliana (Q9C923); Lm, Leishmania major (GenPept gi:13122225); Ap, Aeropyrum pernix (Q9YFZ6); Ss, Sulfolobus sulfataricus (GenPept gi:6015892); Ph, Pyrococcus horikoshii (O58379); Mj, Methanococcus jannaschii (Q58859); Vc, Vibrio cholerae (Q9KM60); Nm, Neisseria meningitidis (Q9JV89); Bs, Bacillus subtilis (ylqF, O31743). (B) Conserved regions G1–G5 in S.cerevisiae sequences that have the same permuted organization of G-motifs as Nog2p (G4 preceding G1) and corresponding motifs in Nog1p and three GTPases of known structure for comparison: Escherichia coli EF-Tu, Thermus thermophilus EF-G and H.sapiens H-ras p21. (C) Wild-type, P324N and S329D Nog2p variants, cloned into the pCM190 vector, were transformed into the GAL::NOG2 strain (LMA159). The empty vector could not support growth of the LMA159 strain under restrictive conditions (glucose medium; data not shown). Transformed cells were streaked on either galactose- or glucose-containing medium.
Similar articles
- The putative GTPases Nog1p and Lsg1p are required for 60S ribosomal subunit biogenesis and are localized to the nucleus and cytoplasm, respectively.
Kallstrom G, Hedges J, Johnson A. Kallstrom G, et al. Mol Cell Biol. 2003 Jun;23(12):4344-55. doi: 10.1128/MCB.23.12.4344-4355.2003. Mol Cell Biol. 2003. PMID: 12773575 Free PMC article. - Nuclear/nucleolar GTPase 2 proteins as a subfamily of YlqF/YawG GTPases function in pre-60S ribosomal subunit maturation of mono- and dicotyledonous plants.
Im CH, Hwang SM, Son YS, Heo JB, Bang WY, Suwastika IN, Shiina T, Bahk JD. Im CH, et al. J Biol Chem. 2011 Mar 11;286(10):8620-8632. doi: 10.1074/jbc.M110.200816. Epub 2011 Jan 4. J Biol Chem. 2011. PMID: 21205822 Free PMC article. - The 60S associated ribosome biogenesis factor LSG1-2 is required for 40S maturation in Arabidopsis thaliana.
Weis BL, Missbach S, Marzi J, Bohnsack MT, Schleiff E. Weis BL, et al. Plant J. 2014 Dec;80(6):1043-56. doi: 10.1111/tpj.12703. Epub 2014 Nov 13. Plant J. 2014. PMID: 25319368 - Pre-ribosomes on the road from the nucleolus to the cytoplasm.
Tschochner H, Hurt E. Tschochner H, et al. Trends Cell Biol. 2003 May;13(5):255-63. doi: 10.1016/s0962-8924(03)00054-0. Trends Cell Biol. 2003. PMID: 12742169 Review. - Getting ready to translate: cytoplasmic maturation of eukaryotic ribosomes.
Panse VG. Panse VG. Chimia (Aarau). 2011;65(10):765-9. doi: 10.2533/chimia.2011.765. Chimia (Aarau). 2011. PMID: 22054128 Review.
Cited by
- Hierarchical recruitment into nascent ribosomes of assembly factors required for 27SB pre-rRNA processing in Saccharomyces cerevisiae.
Talkish J, Zhang J, Jakovljevic J, Horsey EW, Woolford JL Jr. Talkish J, et al. Nucleic Acids Res. 2012 Sep 1;40(17):8646-61. doi: 10.1093/nar/gks609. Epub 2012 Jun 26. Nucleic Acids Res. 2012. PMID: 22735702 Free PMC article. - Evolutionarily conserved role of nucleostemin: controlling proliferation of stem/progenitor cells during early vertebrate development.
Beekman C, Nichane M, De Clercq S, Maetens M, Floss T, Wurst W, Bellefroid E, Marine JC. Beekman C, et al. Mol Cell Biol. 2006 Dec;26(24):9291-301. doi: 10.1128/MCB.01183-06. Epub 2006 Sep 25. Mol Cell Biol. 2006. PMID: 17000755 Free PMC article. - Structural stabilization of GTP-binding domains in circularly permuted GTPases: implications for RNA binding.
Anand B, Verma SK, Prakash B. Anand B, et al. Nucleic Acids Res. 2006 Apr 28;34(8):2196-205. doi: 10.1093/nar/gkl178. Print 2006. Nucleic Acids Res. 2006. PMID: 16648363 Free PMC article. - Serotonin and insulin signaling team up to control growth in Drosophila.
Ruaud AF, Thummel CS. Ruaud AF, et al. Genes Dev. 2008 Jul 15;22(14):1851-5. doi: 10.1101/gad.1700708. Genes Dev. 2008. PMID: 18628391 Free PMC article.
References
- Bourne H.R., Sanders,D.A. and McCormick,F. (1991) The GTPase superfamily: conserved structure and molecular mechanism. Nature, 349, 117–127. - PubMed
- Chandonia J.M. and Karplus,M. (1999) New methods for accurate prediction of protein secondary structure. Proteins, 35, 293–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases