Determinants of activation kinetics in mammalian hyperpolarization-activated cation channels - PubMed (original) (raw)
Determinants of activation kinetics in mammalian hyperpolarization-activated cation channels
T M Ishii et al. J Physiol. 2001.
Abstract
1. The structural basis for the different activation kinetics of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels was investigated with the whole-cell patch clamp technique by using HCN1, HCN4, chimeric channels and mutants in a mammalian expression system (COS-7). 2. The activation time constant of HCN4 was about 40-fold longer than that of HCN1 when compared at -100 mV. 3. In chimeras between HCN1 and HCN4, the region of the S1 transmembrane domain and the exoplasmic S1-S2 linker markedly affected the activation kinetics. The cytoplasmic region between S6 and the cyclic nucleotide-binding domain (CNBD) also significantly affected the activation kinetics. 4. The S1 domain and S1-S2 linker of HCN1 differ from those of HCN4 at eight amino acid residues, and each single point mutation of them changed the activation kinetics less than 2-fold. However, the effects of those mutations were additive and the substitution of the whole S1 and S1-S2 region of HCN1 by that of HCN4 resulted in a 10- to 20-fold slowing. 5. The results indicate that S1 and S1-S2, and S6-CNBD are the crucial components for the activation gating of HCN channels.
Figures
Figure 1. Activation kinetics and voltage-activation curves of HCN1, HCN4 and their deletion mutants
A, schematic representation of mouse HCN1, rabbit HCN4 and their deletion mutants. Black regions represent HCN1 and white regions HCN4 amino acid sequences in this and subsequent figures. B, representative current recordings of HCN1, HCN4 and their deletion mutants (1ΔC and 4ΔNΔC) at 35 °C. The pulse protocol is shown at the top. C, the currents obtained from −100 mV voltage pulse (holding potential = −20 mV) were fitted to single exponential functions to obtain the activation time constants in HCN1 and HCN4 at 35 °C. The dashed lines show the fits and the continuous lines the currents. D, activation time constants as a function of voltage during hyperpolarizing steps for HCN1 (n = 6), 1ΔC (n = 6), HCN4 (n = 9) and 4ΔNΔC (n = 5) at 35 °C. E, activation curves for HCN1, 1ΔC, HCN4 and 4ΔNΔC at 35 °C. The continuous lines represent fits of relative tail current amplitudes to Boltzmann functions. The _V_0.5 and slope factors were −63.1 ± 1.0 mV and 10.6 ± 0.4 mV for HCN1 (n = 8), −63.4 ± 1.1 mV and 9.7 ± 0.4 mV for 1ΔC (n = 8), −62.5 ± 1.4 mV and 5.7 ± 0.2 mV for HCN4 (n = 12), and −72.6 ± 1.4 mV and 5.3 ± 0.3 mV for 4ΔNΔC (n = 5), respectively.
Figure 4. Activation and deactivation kinetics and voltage-activation curves at 35 °C in chimeras with reciprocally replaced S1 and S1–S2 linker regions
A, schematic representation of HCN1-HCN4 chimeras. The exact points of the crossovers for 1–4-1-4A are shown in parentheses. B, representative current recordings of 1–4-1A and 4–1-4A at 35 °C. The pulse protocol is shown in the top. C, activation curves for 1ΔC, 4ΔNΔC, 1–4-1A and 4–1-4A at 35 °C. The continuous lines represent fits of relative tail current amplitudes to Boltzmann functions. The _V_0.5 and slope factors, respectively, were −63.4 ± 1.1 mV and 9.7 ± 0.4 mV for 1ΔC (n = 8), −72.6 ± 1.4 mV and 5.3 ± 0.3 mV for 4ΔNΔC (n = 5), −84.5 ± 1.3 mV and 6.9 ± 0.2 mV for 1–4-1A (n = 9), and −66.8 ± 1.8 mV and 7.0 ± 0.1 mV for 4–1-4A (n = 11). D, activation time constants as a function of voltage during hyperpolarizing steps for 1ΔC (n = 6), 4ΔNΔC (n = 5), 1–4-1A (n = 8), 4–1-4A (n = 5) and 4–1-4-1A (n = 8) at 35 °C.
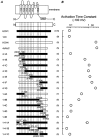
Figure 2. Comparison of activation time constants among HCN1-HCN4 chimeras during −100 mV hyperpolarizing voltage steps
A, schematic representation of HCN1, HCN4, their deletion mutants and HCN1-HCN4 chimeras. The exact points of the crossovers are shown in parentheses for HCN1 top and HCN4 bottom. B, activation time constants during −100 mV hyperpolarizing voltage steps for HCN channels shown in A at 25 °C. The number of experiments in each group is given in parenthesis. The _V_0.5 and slope factors, respectively, were −68.5 ± 2.7 mV and 8.4 ± 1.4 mV for 4–1A (n = 4), −79.5 ± 2.3 mV and 8.9 ± 0.6 mV for 4–1B (n = 6), −81.8 ± 1.2 mV and 6.2 ± 0.4 mV for 4–1C (n = 7), −69.0 ± 1.3 mV and 5.6 ± 0.5 mV for 4–1D (n = 6), −72.0 ± 1.4 mV and 5.8 ± 0.6 mV for 4–1E (n = 7), −75.3 ± 2.3 mV and 6.8 ± 0.8 mV for 4–1F (n = 7), −73.7 ± 2.5 mV and 6.7 ± 0.4 mV for 4–1G (n = 6), −68.6 ± 3.0 mV and 6.6 ± 0.5 mV for 1–4A (n = 6), −75.2 ± 2.1 mV and 8.1 ± 1.0 mV for 1–4B (n = 6), −74.8 ± 1.4 mV and 9.6 ± 0.3 mV for 1–4C (n = 6), −65.8 ± 3.4 mV and 6.9 ± 0.5 mV for 1–4D (n = 4), −61.4 ± 1.6 mV and 9.2 ± 1.7 mV for 1–4E (n = 4), −84.5 ± 1.3 mV and 6.9 ± 0.2 mV for 1–4-1A (n = 9), −64.7 ± 4.8 mV and 11.0 ± 1.2 mV for 1–4-1B (n = 4), and −65.5 ± 2.9 mV and 10.7 ± 0.8 mV for 1–4-1C (n = 4). These factors were measured at 35 °C in slow-activating chimeras (4-1B, 4–1C, 4–1D, 4–1E, 4–1F, 4–1G, 1–4A, 1–4B, and 1–4-1A) and at 25 °C in fast-activating chimeras (4-1A, 1–4 C, 1–4D, 1–4E, 1–4-1B, and 1–4-1C).
Figure 3. Some mutations in S1 and S1-2 linker of HCN1 significantly affected activation time constants during −100 mV hyperpolarizing step
A, comparison of sequences for S1 and S1–S2 linker of mouse HCN1 and rabbit HCN4. Identical residues with respect to the mouse HCN1 sequence are represented by dashes. S1 is indicated by a bar below the sequence. B, activation time constants of 1ΔC mutants during a −100 mV hyperpolarization step at 25 °C. * Significantly different from 1ΔC, P < 0.05. The number of experiments in each group is given in parentheses.
Similar articles
- Tryptophan-scanning mutagenesis in the S1 domain of mammalian HCN channel reveals residues critical for voltage-gated activation.
Ishii TM, Nakashima N, Ohmori H. Ishii TM, et al. J Physiol. 2007 Mar 1;579(Pt 2):291-301. doi: 10.1113/jphysiol.2006.124297. Epub 2006 Dec 21. J Physiol. 2007. PMID: 17185333 Free PMC article. - Regulation of hyperpolarization-activated HCN channel gating and cAMP modulation due to interactions of COOH terminus and core transmembrane regions.
Wang J, Chen S, Siegelbaum SA. Wang J, et al. J Gen Physiol. 2001 Sep;118(3):237-50. doi: 10.1085/jgp.118.3.237. J Gen Physiol. 2001. PMID: 11524455 Free PMC article. - Molecular basis for the different activation kinetics of the pacemaker channels HCN2 and HCN4.
Stieber J, Thomer A, Much B, Schneider A, Biel M, Hofmann F. Stieber J, et al. J Biol Chem. 2003 Sep 5;278(36):33672-80. doi: 10.1074/jbc.M305318200. Epub 2003 Jun 17. J Biol Chem. 2003. PMID: 12813043 - Voltage sensing and activation gating of HCN pacemaker channels.
Chen J, Piper DR, Sanguinetti MC. Chen J, et al. Trends Cardiovasc Med. 2002 Jan;12(1):42-5. doi: 10.1016/s1050-1738(01)00141-4. Trends Cardiovasc Med. 2002. PMID: 11796244 Review. - Cardiac HCN channels: structure, function, and modulation.
Biel M, Schneider A, Wahl C. Biel M, et al. Trends Cardiovasc Med. 2002 Jul;12(5):206-12. doi: 10.1016/s1050-1738(02)00162-7. Trends Cardiovasc Med. 2002. PMID: 12161074 Review.
Cited by
- The enigmatic HCN channels: A cellular neurophysiology perspective.
Mishra P, Narayanan R. Mishra P, et al. Proteins. 2023 Nov 19:prot.26643. doi: 10.1002/prot.26643. Online ahead of print. Proteins. 2023. PMID: 37982354 Free PMC article. Review. - Palmitoylation regulates the magnitude of HCN4-mediated currents in mammalian cells.
Congreve SD, Main A, Butler AS, Gao X, Brown E, Du C, Choisy SC, Cheng H, Hancox JC, Fuller W. Congreve SD, et al. Front Physiol. 2023 Apr 13;14:1163339. doi: 10.3389/fphys.2023.1163339. eCollection 2023. Front Physiol. 2023. PMID: 37123274 Free PMC article. - Whole Exome Sequencing Identifies a Heterozygous Variant in the Cav1.3 Gene CACNA1D Associated with Familial Sinus Node Dysfunction and Focal Idiopathic Epilepsy.
Rinné S, Stallmeyer B, Pinggera A, Netter MF, Matschke LA, Dittmann S, Kirchhefer U, Neudorf U, Opp J, Striessnig J, Decher N, Schulze-Bahr E. Rinné S, et al. Int J Mol Sci. 2022 Nov 17;23(22):14215. doi: 10.3390/ijms232214215. Int J Mol Sci. 2022. PMID: 36430690 Free PMC article. - Bidirectional flow of the funny current (If) during the pacemaking cycle in murine sinoatrial node myocytes.
Peters CH, Liu PW, Morotti S, Gantz SC, Grandi E, Bean BP, Proenza C. Peters CH, et al. Proc Natl Acad Sci U S A. 2021 Jul 13;118(28):e2104668118. doi: 10.1073/pnas.2104668118. Proc Natl Acad Sci U S A. 2021. PMID: 34260402 Free PMC article. - Isoform-specific regulation of HCN4 channels by a family of endoplasmic reticulum proteins.
Peters CH, Myers ME, Juchno J, Haimbaugh C, Bichraoui H, Du Y, Bankston JR, Walker LA, Proenza C. Peters CH, et al. Proc Natl Acad Sci U S A. 2020 Jul 28;117(30):18079-18090. doi: 10.1073/pnas.2006238117. Epub 2020 Jul 9. Proc Natl Acad Sci U S A. 2020. PMID: 32647060 Free PMC article.
References
- Chen J, Mitcheson JS, Lin M, Sanguinetti MC. Functional roles of charged residues in the putative voltage sensor of the HCN2 pacemaker channel. Journal of Biological Chemistry. 2000;275:36465–36471. - PubMed
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annual Review of Physiology. 1993;55:455–472. - PubMed
- Ishii TM, Takano M, Xie L-H, Noma A, Ohmori H. Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. Journal of Biological Chemistry. 1999;274:12835–12839. - PubMed
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials