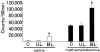The effects of dentate granule cell destruction on behavioural activity and Fos protein expression induced by systemic methamphetamine in rats - PubMed (original) (raw)
. 2001 Dec;134(7):1411-8.
doi: 10.1038/sj.bjp.0704370.
M Iyo, H Matsumoto, M Kawai, K Suzuki, Y Iwata, T Won, T Tsukamoto, Y Sekine, M Sakanoue, K Hashimoto, Y Ohashi, N Takei, N Mori
Affiliations
- PMID: 11724746
- PMCID: PMC1573072
- DOI: 10.1038/sj.bjp.0704370
The effects of dentate granule cell destruction on behavioural activity and Fos protein expression induced by systemic methamphetamine in rats
K Tani et al. Br J Pharmacol. 2001 Dec.
Abstract
1. We destroyed dentate granule cells unilaterally or bilaterally by means of intrahippocampal injection of colchicine in rats. Subsequently, we observed behavioural changes following the intraperitoneal injection of 2 mg kg(-1) methamphetamine or saline, in addition to quantitatively assessing Fos protein expression in several brain regions, including the medial prefrontal cortex, cingulate cortex, piriform cortex, dorsal striatum, and nucleus accumbens. 2. Bilaterally lesioned animals, when administered saline, showed a marked increase in locomotor activity compared with those of non-lesioned animals. With respect to the methamphetamine response, bilateral destruction resulted in a marked enhancement of locomotor activity, while the unilateral destruction led to a marked increase in rotation predominantly contralateral to the lesioned side, with no identifiable change in locomotor activity. 3. Bilaterally lesioned animals, when administered saline and having undergone an immunohistological examination, showed a marked increase in Fos expression in both sides of the nucleus accumbens. Bilaterally lesioned animals administered methamphetamine showed a marked increase in Fos expression in the right and left sides of all regions tested. Unilaterally lesioned animals administered methamphetamine showed a significant and bilateral enhancement in Fos expression in the medial prefrontal and cingulate cortices, and a marked and unilateral (ipsilateral to the lesioned side) enhancement of Fos protein in the piriform cortex, dorsal striatum, and nucleus accumbens. 4. The present findings suggest that dentate granule cells regulate methamphetamine-associated behavioural changes through the function of widespread areas of the brain, mostly the nucleus accumbens.
Figures
Figure 1
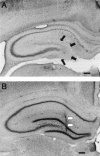
Morphological effects of intrahippocampal colchicine (A) or saline (B). Coronal sections of the dorsal hippocampus stained with cresyl violet are shown. The granule cells are almost completely destroyed by colchicine (black arrowheads) (A). The white arrowheads show the insertion track of the cannulae used for injecting saline (B). The calibration bar is 500 μm.
Figure 2
Amount of locomotor activity after saline or methamphetamine administration in the C, BL, and UL group animals. The amount of locomotion in the BL group was significantly larger than that for the C (*P<0.001) and UL group (*P<0.001) after saline administration, while the latter two groups showed no significant difference (P_=0.88). After methamphetamine administration, the value for the BL group was markedly larger than that for the C (†_P<0.001) and UL group (†P<0.001), while there was no significant difference in locomotion between the latter two groups (_P_=0.23).
Figure 3
The induction of Fos protein in the dorsal striatum after saline (A) or methamphetamine administration (B), each from the BL group. The calibration bar is 100 μm.
Figure 4
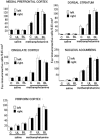
The number of Fos-positive cells in the medial prefrontal cortex, cingulate cortex, piriform cortex, dorsal striatum, and nucleus accumbens following saline or methamphetamine administration. The white bars are for the left sides in the regions and the black bars are for the right sides. Error bars indicate s.e.mean. See also Tables 2A and B for post hoc pair comparisons.
Similar articles
- The effects of dentate granule cell destruction on behavioral activity and Fos protein expression induced by systemic MDMA in rats.
Won M, Minabe Y, Tani K, Suzuki K, Kawai M, Sekine Y, Ashby CR Jr, Takei N, Mori N. Won M, et al. Neurosci Res. 2003 Jun;46(2):153-60. doi: 10.1016/s0168-0102(03)00041-5. Neurosci Res. 2003. PMID: 12767478 - Medial prefrontal cortex and nucleus accumbens core are involved in retrieval of the methamphetamine-associated memory.
Chiang CY, Cherng CG, Lai YT, Fan HY, Chuang JY, Kao GS, Chang WT, Yu L. Chiang CY, et al. Behav Brain Res. 2009 Jan 30;197(1):24-30. doi: 10.1016/j.bbr.2008.07.030. Epub 2008 Aug 5. Behav Brain Res. 2009. PMID: 18722478 - Anatomical substrates for the discriminative stimulus effects of methamphetamine in rats.
Nakajima A, Yamada K, He J, Zeng N, Nitta A, Nabeshima T. Nakajima A, et al. J Neurochem. 2004 Oct;91(2):308-17. doi: 10.1111/j.1471-4159.2004.02705.x. J Neurochem. 2004. PMID: 15447664 - Activation of feeding-related neural circuitry after unilateral injections of muscimol into the nucleus accumbens shell.
Stratford TR. Stratford TR. Brain Res. 2005 Jun 28;1048(1-2):241-50. doi: 10.1016/j.brainres.2005.05.002. Brain Res. 2005. PMID: 15921658 - A novel pharmacological concept in an animal model of psychosis.
Dawirs RR, Teuchert-Noodt G. Dawirs RR, et al. Acta Psychiatr Scand Suppl. 2001;(408):10-7. doi: 10.1034/j.1600-0447.2001.104s408010.x. Acta Psychiatr Scand Suppl. 2001. PMID: 11730069 Review.
Cited by
- Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction.
Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Noonan MA, et al. J Neurosci. 2010 Jan 6;30(1):304-15. doi: 10.1523/JNEUROSCI.4256-09.2010. J Neurosci. 2010. PMID: 20053911 Free PMC article. - Effects of a 33-ion sequential beam galactic cosmic ray analog on male mouse behavior and evaluation of CDDO-EA as a radiation countermeasure.
Kiffer FC, Luitel K, Tran FH, Patel RA, Guzman CS, Soler I, Xiao R, Shay JW, Yun S, Eisch AJ. Kiffer FC, et al. Behav Brain Res. 2022 Feb 15;419:113677. doi: 10.1016/j.bbr.2021.113677. Epub 2021 Nov 21. Behav Brain Res. 2022. PMID: 34818568 Free PMC article. - Irradiation in adulthood as a new model of schizophrenia.
Iwata Y, Suzuki K, Wakuda T, Seki N, Thanseem I, Matsuzaki H, Mamiya T, Ueki T, Mikawa S, Sasaki T, Suda S, Yamamoto S, Tsuchiya KJ, Sugihara G, Nakamura K, Sato K, Takei N, Hashimoto K, Mori N. Iwata Y, et al. PLoS One. 2008 May 28;3(5):e2283. doi: 10.1371/journal.pone.0002283. PLoS One. 2008. PMID: 18509473 Free PMC article. - Comprehensive behavioral phenotyping of Ts65Dn mouse model of Down syndrome: activation of β1-adrenergic receptor by xamoterol as a potential cognitive enhancer.
Faizi M, Bader PL, Tun C, Encarnacion A, Kleschevnikov A, Belichenko P, Saw N, Priestley M, Tsien RW, Mobley WC, Shamloo M. Faizi M, et al. Neurobiol Dis. 2011 Aug;43(2):397-413. doi: 10.1016/j.nbd.2011.04.011. Epub 2011 Apr 17. Neurobiol Dis. 2011. PMID: 21527343 Free PMC article. - Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration.
Recinto P, Samant AR, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O, Mandyam CD. Recinto P, et al. Neuropsychopharmacology. 2012 Apr;37(5):1275-87. doi: 10.1038/npp.2011.315. Epub 2011 Dec 28. Neuropsychopharmacology. 2012. PMID: 22205547 Free PMC article.
References
- ANTONIOU K., KAFETZOPOULOS E. Behavioural effects of amphetamine and apomorphine after striatal lesions in the rat. Phamacol. Biochem. Behav. 1992;43:705–722. - PubMed
- BERTRAM E.H., ZHANG D.X., MANGAN P., FOUNTAIN N., REMPE D. Functional anatomy of limbic epilepsy: a proposal for central synchronization of a diffusely hyperexcitable network. Epilepsy Res. 1998;32:194–205. - PubMed
- CARBONI E., IMPERATO A., PEREZZANI L., DI CHIARA G. Amphetamine, cocaine phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. - PubMed
- CHRISTIE M.J., SUMMERS R.J., STEPHENSON J.A., COOK C.J., BEART P.M. Excitatory amino acid projections to the nucleus accumbens septi in the rat: a retrograde transport study utilizing D[3H]aspartate and [3H]GABA. Neuroscience. 1987;22:425–439. - PubMed
- COLLE L.M., WISE R.A. Circling induced by intra-accumbens amphetamine injections. Psychopharmacol. 1991;105:157–161. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical