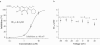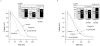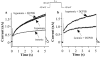DCPIB is a novel selective blocker of I(Cl,swell) and prevents swelling-induced shortening of guinea-pig atrial action potential duration - PubMed (original) (raw)
DCPIB is a novel selective blocker of I(Cl,swell) and prevents swelling-induced shortening of guinea-pig atrial action potential duration
N Decher et al. Br J Pharmacol. 2001 Dec.
Abstract
1. We identified the ethacrynic-acid derivative DCPIB as a potent inhibitor of I(Cl,swell), which blocks native I(Cl,swell) of calf bovine pulmonary artery endothelial (CPAE) cells with an IC(50) of 4.1 microM. Similarly, 10 microM DCPIB almost completely inhibited the swelling-induced chloride conductance in Xenopus oocytes and in guinea-pig atrial cardiomyocytes. Block of I(Cl,swell) by DCPIB was fully reversible and voltage independent. 2. DCPIB (10 microM) showed selectivity for I(Cl,swell) and had no significant inhibitory effects on I(Cl,Ca) in CPAE cells, on chloride currents elicited by several members of the CLC-chloride channel family or on the human cystic fibrosis transmembrane conductance regulator (hCFTR) after heterologous expression in Xenopus oocytes. DCPIB (10 microM) also showed no significant inhibition of several native anion and cation currents of guinea pig heart like I(Cl,PKA), I(Kr), I(Ks), I(K1), I(Na) and I(Ca). 3. In all atrial cardiomyocytes (n=7), osmotic swelling produced an increase in chloride current and a strong shortening of the action potential duration (APD). Both swelling-induced chloride conductance and AP shortening were inhibited by treatment of swollen cells with DCPIB (10 microM). In agreement with the selectivity for I(Cl,swell), DCPIB did not affect atrial APD under isoosmotic conditions. 4. Preincubation of atrial cardiomyocytes with DCPIB (10 microM) completely prevented both the swelling-induced chloride currents and the AP shortening but not the hypotonic cell swelling. 5. We conclude that swelling-induced AP shortening in isolated atrial cells is mainly caused by activation of I(Cl,swell). DCPIB therefore is a valuable pharmacological tool to study the role of I(Cl,swell) in cardiac excitability under pathophysiological conditions leading to cell swelling.
Figures
Figure 1
Chemical structure and dose response curve of DCPIB on _I_Cl,swell in CPAE cells. (a) DCPIB blocked _I_Cl,swell in CPAE cells with a half-maximal concentration (IC50) of 4.1 μ
M
at −80 mV. Inhibition values were calculated without subtraction of the basal current. If the basal non-DCPIB sensitive current is subtracted the resulting IC50 is 2.5 μ
M
at −80 mV. (b) IC50 values of DCPIB determined at different voltages without subtraction of the basal current (n.d.=not determined).
Figure 2
Effects of DCPIB on native _I_Cl,swell and _I_Cl,Ca currents. (a) _I_Cl,swell current traces from CPAE cells after hypotonic solution was applied for 3 – 5 min in the absence and (b) in the presence of 10 μ
M
DCPIB. (c) I – V curves of _I_Cl,swell obtained with the illustrated step pulse protocol, in isotonic medium (isotonic), after cell swelling in hypotonic medium (hypotonic) and following the treatment of swollen cells with 10 μ
M
DCPIB (hypotonic+10 μ
M
DCPIB). (d) _I_Cl,Ca currents recorded from CPAE cells in response to voltage steps applied every 3 s from a holding potential of −50 mV. (e) _I_Cl,Ca currents in the presence of 10 μ
M
DCPIB. (f) I – V relationships from currents in (d) and (e). Currents were measured at the end of the voltage steps. (g) _I_Cl,swell currents of guinea-pig atrial cardiomyocytes. Hypotonic solution was applied for 5 – 10 min. Atrial currents were measured starting from a holding potential of 0 mV using the illustrated step pulse protocol. (h) _I_Cl,swell currents in presence of 10 μ
M
DCPIB in the hypotonic bath solution. (i) Corresponding I – V curves.
Figure 3
Photographs of guinea-pig atrial cardiomyocytes during whole cell patch clamp recording. Cell width was analysed by averaging the cell width at three different places of the myocyte. (a) In isotonic solution. (b) Following 10 min of hypotonic perfusion and (c) after perfusion (5 min) with 10 μ
M
DCPIB of swollen cells from (b). The figure illustrates that DCPIB did not decrease the cell swelling during hypotonicity. (d) Atrial cardiomyocyte in isotonic solution and (e) after 5 min of preincubation with 10 μ
M
DCPIB and (f) after perfusion with hypotonic medium containing 10 μ
M
DCPIB. Preincubation with DCPIB did not inhibit subsequent cell swelling in hypotonic medium.
Figure 4
Effects of 10 μ
M
DCPIB on CLC-chloride channels and on hCFTR expressed in Xenopus oocytes. hCFTR was activated by 1 m
M
IBMX and 10 μ
M
forskolin. Currents were elicited starting from a holding potential of −30 mV according to the illustrated step protocols. Original current traces show control currents. I – V relationships were determined from control currents and currents in the presence of DCPIB.
Figure 5
Influence of 10 μ
M
DCPIB on currents of guinea-pig cardiomyocytes. Holding potential in whole cell patches was routinely −80 mV, but 0 mV for measurement of _I_Cl,PKA. Inhibition of _I_Kr was analysed from the tail currents at −40 mV. _I_Ks inhibition data were obtained from the steady state current at +60 mV. To show that the used voltage protocols (Bosch et al., 1998) can separate _I_Kr and _I_Ks we tested their inhibition by 0.2 μ
M
dofetilide and 1.0 μ
M
HMR 1556, respectively. _I_K1 inhibition values were obtained at −100 mV. _I_Na inward currents were analysed by stepping from the holding potential to −30 mV for 20 ms, with a frequency of 1 Hz. _I_Ca was studied by a step protocol to analyse its peak inward current. The pulses were applied every 15 s to minimize rundown. _I_Cl,PKA was activated by isoprenaline (1 μ
M
) or alternatively IBMX (500 μ
M
).
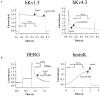
Figure 6
Effects of 10 μ
M
DCPIB on human Kv and delayed rectifier channels, which encode the most important repolarizing K+ currents in the human heart. The channels were expressed in Xenopus oocytes. Holding potential was −80 mV. (a) Current traces of hKv1.5 (_I_Kur) and hKv4.3 (_I_to1) before (control) and subsequent to the addition of 10 μ
M
DCPIB. (b) Original current traces of HERG (_I_Kr) and hminK (_I_Ks) injected Xenopus oocytes in ND96 (control) and in the presence of 10 μ
M
DCPIB.
Figure 7
Influence of hypotonicity and 10 μ
M
DCPIB on atrial action potentials. (a) Individual action potential recordings of control cells (isotonic) were compared with AP traces from swollen cells (hypotonic), obtained by perfusion of cells with hypotonic medium for 5 – 10 min. Hypotonic solution caused a marked reduction in APD. Subsequent addition of 10 μ
M
DCPIB reversed APD shortening (hypotonic+10 μ
M
DCPIB). Attenuation of the peak voltage under hypotonic medium was only observed in two out of seven cells. Inset: The histogram illustrates the results displayed in Table 3a. Action potential durations were normalized and expressed as percentage of the isotonic APD. **P<0.01 compared with isotonic values. (b) Prevention of swelling-induced atrial action potential shortening by 10 μ
M
DCPIB. Individual action potentials were recorded from atrial myocytes after perfusion with 10 μ
M
DCPIB in isotonic solution for 5 min. DCPIB did not affect APD under isotonic conditions (compare isotonic vs isotonic+10 μ
M
DCPIB). Subsequent treatment with hypotonic medium containing 10 μ
M
DCPIB for 5 – 10 min did not result in APD shortening (hypotonic+10 μ
M
DCPIB). Inset: The histogram illustrates the results displayed in Table 3b. Action potential durations were normalized to isotonic conditions and expressed as percentage of the isotonic values. *P<0.05 compared with isotonic values.
Figure 8
Effects of DCPIB on swelling-enhanced _I_Ks currents. _I_Ks was evoked by stepping from a holding potential of −40 to +60 mV for 5 s under Ca2+-free conditions. (a) Representative _I_Ks current traces in isotonic solution (isotonic), after cell swelling caused by hypotonic perfusion (hypotonic) and after addition of 10 μ
M
DCPIB to the hypotonic solution (hypotonic+DCPIB). (b) Representative _I_Ks current traces in the presence of 10 μ
M
DCPIB in the isotonic solution (isotonic+DCPIB) and after cell swelling in the presence of DCPIB (hypotonic+DCPIB).
Similar articles
- Swelling-induced Cl- current in guinea-pig atrial myocytes: inhibition by glibenclamide.
Sakaguchi M, Matsuura H, Ehara T. Sakaguchi M, et al. J Physiol. 1997 Nov 15;505 ( Pt 1)(Pt 1):41-52. doi: 10.1111/j.1469-7793.1997.041bc.x. J Physiol. 1997. PMID: 9409470 Free PMC article. - The ICl,swell inhibitor DCPIB blocks Kir channels that possess weak affinity for PIP2.
Deng W, Mahajan R, Baumgarten CM, Logothetis DE. Deng W, et al. Pflugers Arch. 2016 May;468(5):817-24. doi: 10.1007/s00424-016-1794-9. Epub 2016 Feb 2. Pflugers Arch. 2016. PMID: 26837888 Free PMC article. - Chlorotoxin does not inhibit volume-regulated, calcium-activated and cyclic AMP-activated chloride channels.
Maertens C, Wei L, Tytgat J, Droogmans G, Nilius B. Maertens C, et al. Br J Pharmacol. 2000 Feb;129(4):791-801. doi: 10.1038/sj.bjp.0703102. Br J Pharmacol. 2000. PMID: 10683204 Free PMC article. - Role of cardiac chloride currents in changes in action potential characteristics and arrhythmias.
Hiraoka M, Kawano S, Hirano Y, Furukawa T. Hiraoka M, et al. Cardiovasc Res. 1998 Oct;40(1):23-33. doi: 10.1016/s0008-6363(98)00173-4. Cardiovasc Res. 1998. PMID: 9876314 Review. - Chloride channels: a molecular perspective.
Jentsch TJ. Jentsch TJ. Curr Opin Neurobiol. 1996 Jun;6(3):303-10. doi: 10.1016/s0959-4388(96)80112-7. Curr Opin Neurobiol. 1996. PMID: 8794080 Review.
Cited by
- Taurine release by astrocytes modulates osmosensitive glycine receptor tone and excitability in the adult supraoptic nucleus.
Choe KY, Olson JE, Bourque CW. Choe KY, et al. J Neurosci. 2012 Sep 5;32(36):12518-27. doi: 10.1523/JNEUROSCI.1380-12.2012. J Neurosci. 2012. PMID: 22956842 Free PMC article. - Glycine release from radial cells modulates the spontaneous activity and its propagation during early spinal cord development.
Scain AL, Le Corronc H, Allain AE, Muller E, Rigo JM, Meyrand P, Branchereau P, Legendre P. Scain AL, et al. J Neurosci. 2010 Jan 6;30(1):390-403. doi: 10.1523/JNEUROSCI.2115-09.2010. J Neurosci. 2010. PMID: 20053920 Free PMC article. - Hypertonicity-induced cation channels rescue cells from staurosporine-elicited apoptosis.
Numata T, Sato K, Okada Y, Wehner F. Numata T, et al. Apoptosis. 2008 Jul;13(7):895-903. doi: 10.1007/s10495-008-0220-y. Apoptosis. 2008. PMID: 18478334 Free PMC article. - Influence of WNK3 on intracellular chloride concentration and volume regulation in HEK293 cells.
Cruz-Rangel S, Gamba G, Ramos-Mandujano G, Pasantes-Morales H. Cruz-Rangel S, et al. Pflugers Arch. 2012 Sep;464(3):317-30. doi: 10.1007/s00424-012-1137-4. Epub 2012 Aug 3. Pflugers Arch. 2012. PMID: 22864523
References
- AKIYAMA T., FOZZARD H.A. Influence of potassium ions and osmolality on the resting membrane potential of rabbit ventricular papillary muscle with estimation of the activity and the activity coefficient of internal potassium. Circ. Res. 1975;37:621–629. - PubMed
- ARELLANO R.O., WOODWARD R.M., MILEDI R. Ion channels and membrane receptors in follicle-enclosed Xenopus oocytes. New York: Plenum Press; Ion Channels. 1996;vol. 4:203–259. - PubMed
- BEAR C.E., DUGUAY F., NAISMITH A.L., KARTNER N., HANRAHAN J.W., RIORDAN J.R. Cl− channel activity in Xenopus oocytes expressing the cystic fibrosis gene. J. Biol. Chem. 1991;266:19142–19145. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous