A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast - PubMed (original) (raw)
A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast
E V Pavlov et al. J Cell Biol. 2001.
Abstract
During apoptosis, proapoptotic factors are released from mitochondria by as yet undefined mechanisms. Patch-clamping of mitochondria and proteoliposomes formed from mitochondrial outer membranes of mammalian (FL5.12) cells has uncovered a novel ion channel whose activity correlates with onset of apoptosis. The pore diameter inferred from the largest conductance state of this channel is approximately 4 nm, sufficient to allow diffusion of cytochrome c and even larger proteins. The activity of the channel is affected by Bcl-2 family proteins in a manner consistent with their pro- or antiapoptotic properties. Thus, the channel activity correlates with presence of proapoptotic Bax in the mitochondrial outer membrane and is absent in mitochondria from cells overexpressing antiapoptotic Bcl-2. Also, a similar channel activity is found in mitochondrial outer membranes of yeast expressing human Bax. These findings implicate this channel, named mitochondrial apoptosis-induced channel, as a candidate for the outer-membrane pore through which cytochrome c and possibly other factors exit mitochondria during apoptosis.
Figures
Figure 1.
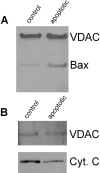
Proteoliposomes enriched with Bax fail to retain cytochrome c. (A) Immunoblots show Bax (but not VDAC) levels are higher (5–10-fold) in outer membranes purified from mitochondria isolated from FL5.12 cells 12 h after IL-3 withdrawal (i.e., apoptotic cells) than from normal cells (i.e., control). Silver-stained gels and Western blots with antibodies against cytochrome oxidase subunit IV indicate equivalent content of overall protein and low contamination by inner membranes for both preparations (data not shown). (B) Immunoblots show that less cytochrome c is present in salt-washed proteoliposomes made from mitochondrial outer membranes of apoptotic cells than of normal cells (control).
Figure 2.
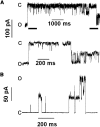
Detection and characterization of MAC activity and comparison with VDAC and TOM channels. (A) Single-channel current traces reveal MAC activity with 3 nS peak transition size and predominant transitions of 1.5 nS at −40 mV. Lower current traces are time-expanded regions of the top trace (indicated by heavy lines). (B) Current trace of MAC at 80 mV shows 2 nS peak conductance with multiple 1 nS transitions. (C) Current traces of single channels at 20 mV show MAC character is distinct from TOM channel and VDAC. O and C indicate open and closed conductance levels. Patching media was symmetrical 150 mM KCl, 5 mM Hepes-koh/ pH 7.4. Current traces, low pass filtered at 2 kHz (5 kHz sampling), were obtained using patch-clamp procedures as described in Materials and methods. (D) Histograms show the frequency of detecting MAC in independent patches (n) from proteoliposomes prepared with outer membranes purified from mitochondria isolated before (closed, +IL-3 control) or 12 h after IL-3 withdrawal (open, −IL-3 apoptotic) from FL5.12 cells that were parental or expressing incompetent (Bcl-2 mutant) or functional Bcl-2 (Bcl-2). Statistical difference (P < 0.05) between pairs was determined by Fischer's exact statistical test (Fisher, 1935) and indicated by asterisks.
Figure 2.
Detection and characterization of MAC activity and comparison with VDAC and TOM channels. (A) Single-channel current traces reveal MAC activity with 3 nS peak transition size and predominant transitions of 1.5 nS at −40 mV. Lower current traces are time-expanded regions of the top trace (indicated by heavy lines). (B) Current trace of MAC at 80 mV shows 2 nS peak conductance with multiple 1 nS transitions. (C) Current traces of single channels at 20 mV show MAC character is distinct from TOM channel and VDAC. O and C indicate open and closed conductance levels. Patching media was symmetrical 150 mM KCl, 5 mM Hepes-koh/ pH 7.4. Current traces, low pass filtered at 2 kHz (5 kHz sampling), were obtained using patch-clamp procedures as described in Materials and methods. (D) Histograms show the frequency of detecting MAC in independent patches (n) from proteoliposomes prepared with outer membranes purified from mitochondria isolated before (closed, +IL-3 control) or 12 h after IL-3 withdrawal (open, −IL-3 apoptotic) from FL5.12 cells that were parental or expressing incompetent (Bcl-2 mutant) or functional Bcl-2 (Bcl-2). Statistical difference (P < 0.05) between pairs was determined by Fischer's exact statistical test (Fisher, 1935) and indicated by asterisks.
Figure 2.
Detection and characterization of MAC activity and comparison with VDAC and TOM channels. (A) Single-channel current traces reveal MAC activity with 3 nS peak transition size and predominant transitions of 1.5 nS at −40 mV. Lower current traces are time-expanded regions of the top trace (indicated by heavy lines). (B) Current trace of MAC at 80 mV shows 2 nS peak conductance with multiple 1 nS transitions. (C) Current traces of single channels at 20 mV show MAC character is distinct from TOM channel and VDAC. O and C indicate open and closed conductance levels. Patching media was symmetrical 150 mM KCl, 5 mM Hepes-koh/ pH 7.4. Current traces, low pass filtered at 2 kHz (5 kHz sampling), were obtained using patch-clamp procedures as described in Materials and methods. (D) Histograms show the frequency of detecting MAC in independent patches (n) from proteoliposomes prepared with outer membranes purified from mitochondria isolated before (closed, +IL-3 control) or 12 h after IL-3 withdrawal (open, −IL-3 apoptotic) from FL5.12 cells that were parental or expressing incompetent (Bcl-2 mutant) or functional Bcl-2 (Bcl-2). Statistical difference (P < 0.05) between pairs was determined by Fischer's exact statistical test (Fisher, 1935) and indicated by asterisks.
Figure 3.
Bax forms high conductance channels with multiple conductance levels. Current traces show hBax channel activity at various voltages had transitions ranging from 0.15–2.0 nS. Purified oligomeric hBax (34 ng/μl in the pipette tip) self-inserted into patches excised from liposomes originally formed without protein. Other conditions are as in Figs. 1 and 2.
Figure 4.
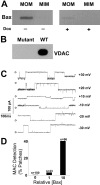
MAC activity induced by Bax expression in yeast. VDAC-less yeast carrying a human Bax plasmid were grown either in the presence of doxycyclin to repress expression or in its absence for 16 h to enable expression. (A) Immunoblot shows Bax localized to mitochondrial outer (MOM) and not inner (MIM) membranes. Densitometry (Unscanit program) indicated Bax levels were ∼10-fold higher in outer membranes of yeast grown without rather than with doxycyclin (Dox). (B) Immunoblot of mitochondria (15 μg) confirms that this strain (Mutant) is VDAC-deficient compared with the wild-type strain (WT). (C) Current traces of MAC were recorded from patches excised from proteoliposomes containing mitochondrial outer membranes purified from yeast expressing human Bax. (D) Histograms show the frequency of detecting MAC in independent patches (n) from proteoliposomes prepared with outer membranes purified from mitochondria with different Bax content. Wild-type yeast do not express Bax (0 level). The Bax levels of Western blot shown in A were normalized to outer membranes of DBY747/b5 yeast grown in the presence of doxycyclin. MAC detection was statistically different (P < 0.001) before and after induction of Bax expression. Other conditions are as in Figs. 1 and 2.
Similar articles
- Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC.
Shimizu S, Narita M, Tsujimoto Y. Shimizu S, et al. Nature. 1999 Jun 3;399(6735):483-7. doi: 10.1038/20959. Nature. 1999. PMID: 10365962 - Mouse uterine epithelial apoptosis is associated with expression of mitochondrial voltage-dependent anion channels, release of cytochrome C from mitochondria, and the ratio of Bax to Bcl-2 or Bcl-X.
Takagi-Morishita Y, Yamada N, Sugihara A, Iwasaki T, Tsujimura T, Terada N. Takagi-Morishita Y, et al. Biol Reprod. 2003 Apr;68(4):1178-84. doi: 10.1095/biolreprod.102.007997. Epub 2002 Oct 30. Biol Reprod. 2003. PMID: 12606449 - Bax and Bcl-xL independently regulate apoptotic changes of yeast mitochondria that require VDAC but not adenine nucleotide translocator.
Shimizu S, Shinohara Y, Tsujimoto Y. Shimizu S, et al. Oncogene. 2000 Sep 7;19(38):4309-18. doi: 10.1038/sj.onc.1203788. Oncogene. 2000. PMID: 10980606 - The voltage-dependent anion channel: an essential player in apoptosis.
Tsujimoto Y, Shimizu S. Tsujimoto Y, et al. Biochimie. 2002 Feb-Mar;84(2-3):187-93. doi: 10.1016/s0300-9084(02)01370-6. Biochimie. 2002. PMID: 12022949 Review. - Control of mitochondrial permeability by Bcl-2 family members.
Sharpe JC, Arnoult D, Youle RJ. Sharpe JC, et al. Biochim Biophys Acta. 2004 Mar 1;1644(2-3):107-13. doi: 10.1016/j.bbamcr.2003.10.016. Biochim Biophys Acta. 2004. PMID: 14996495 Review.
Cited by
- GSK3β Inhibition Is the Molecular Pivot That Underlies the Mir-210-Induced Attenuation of Intrinsic Apoptosis Cascade during Hypoxia.
Marwarha G, Røsand Ø, Slagsvold KH, Høydal MA. Marwarha G, et al. Int J Mol Sci. 2022 Aug 19;23(16):9375. doi: 10.3390/ijms23169375. Int J Mol Sci. 2022. PMID: 36012628 Free PMC article. - Reflections on VDAC as a voltage-gated channel and a mitochondrial regulator.
Mannella CA, Kinnally KW. Mannella CA, et al. J Bioenerg Biomembr. 2008 Jun;40(3):149-55. doi: 10.1007/s10863-008-9143-0. J Bioenerg Biomembr. 2008. PMID: 18648913 Review. - Assembly of the mitochondrial apoptosis-induced channel, MAC.
Martinez-Caballero S, Dejean LM, Kinnally MS, Oh KJ, Mannella CA, Kinnally KW. Martinez-Caballero S, et al. J Biol Chem. 2009 May 1;284(18):12235-45. doi: 10.1074/jbc.M806610200. Epub 2009 Mar 4. J Biol Chem. 2009. PMID: 19261612 Free PMC article. - Paraquat Induces Cell Death Through Impairing Mitochondrial Membrane Permeability.
Huang CL, Chao CC, Lee YC, Lu MK, Cheng JJ, Yang YC, Wang VC, Chang WC, Huang NK. Huang CL, et al. Mol Neurobiol. 2016 May;53(4):2169-88. doi: 10.1007/s12035-015-9198-y. Epub 2015 May 7. Mol Neurobiol. 2016. PMID: 25947082 - Mitochondrial carrier homolog 2: a clue to cracking the BCL-2 family riddle?
Gross A. Gross A. J Bioenerg Biomembr. 2005 Jun;37(3):113-9. doi: 10.1007/s10863-005-6222-3. J Bioenerg Biomembr. 2005. PMID: 16167168 Review.
References
- Antonsson, B., F. Conti, A. Ciavatta, S. Montessuit, S. Lewis, I. Martinou, L. Bernasconi, A. Bernard, J.J. Mermod, G. Mazzei, et al. 1997. Inhibition of Bax channel-forming activity by Bcl-2. Science. 277:370–372. - PubMed
- Antonsson, B., S. Montessuit, B. Sanchez, and J.C. Martinou. 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276:11615–11623. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials