Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo - PubMed (original) (raw)
Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo
J Priller et al. J Cell Biol. 2001.
Abstract
The versatility of stem cells has only recently been fully recognized. There is evidence that upon adoptive bone marrow (BM) transplantation (BMT), donor-derived cells can give rise to neuronal phenotypes in the brains of recipient mice. Yet only few cells with the characteristic shape of neurons were detected 1-6 mo post-BMT using transgenic or newborn mutant mice. To evaluate the potential of BM to generate mature neurons in adult C57BL/6 mice, we transferred the enhanced green fluorescent protein (GFP) gene into BM cells using a murine stem cell virus-based retroviral vector. Stable and high level long-term GFP expression was observed in mice transplanted with the transduced BM. Engraftment of GFP-expressing cells in the brain was monitored by intravital microscopy. In a long-term follow up of 15 mo post-BMT, fully developed Purkinje neurons were found to express GFP in both cerebellar hemispheres and in all chimeric mice. GFP-positive Purkinje cells were also detected in BM chimeras from transgenic mice that ubiquitously express GFP. Based on morphologic criteria and the expression of glutamic acid decarboxylase, the newly generated Purkinje cells were functional.
Figures
Figure 1.
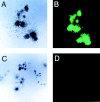
Retroviral-mediated transfer of the GFP gene into hematopoietic cells. Adult BM cells were transduced with a murine stem cell virus–based retroviral vector containing the GFP marker gene. Cells were subsequently plated in methylcellulose supplemented with hematopoietic growth factors, and analyzed for GFP expression after 7 d in culture. The top two panels show identical GFP-transduced hematopoietic colonies, visualized separately under phase-contrast (A) and fluorescence (B) microscopy. The bottom two panels demonstrate nontransduced hematopoietic colonies under phase-contrast (C) and fluorescence (D) microscopy. Note the lack of background fluorescence in (D).
Figure 2.
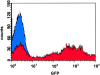
Reconstitution of hematopoiesis with GFP-marked peripheral blood cell progeny in myeloablated mice. Flow cytometric analysis of GFP expression was performed in peripheral blood leukocytes of lethally irradiated mice 8 mo after transplantation of GFP-expressing BM cells (red) and in leukocytes of control mice that did not receive GFP-labeled BM (blue). Approximately 70% of white blood cells were stably expressing the fluorophore in GFP-BM chimeras. All myeloid and lymphocytic populations were labeled, but the levels of GFP expression varied within lineages. Highest expression was observed in monocytes/macrophages and granulocytes (unpublished data).
Figure 3.
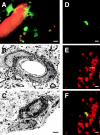
Detection of GFP-expressing cells in the brains of BM chimeras. 4 wk after transplantation of GFP-expressing BM cells, a closed cranial window was implanted in anesthetized mice. After intravenous injection of the dye rhodamine B dextran to contrast the lumen of cerebral vessels, GFP-expressing cells were detected in vivo using laser-scanning microscopy through the cranial window (A). Some extraluminal GFP-positive cells extended short cellular processes and showed a punctate rhodamine staining from dye uptake. These cells were located in the perivascular space (the basement membrane is indicated by arrows), as demonstrated by immunoelectron microscopy for GFP (B). Note that endothelia (E) did not express GFP. In C, a donor-derived cell is visible that has crossed the blood brain barrier and is located in the brain parenchyma. 13 wk post-BMT, a GFP-expressing cell in the olfactory bulb (D) demonstrates NeuN-immunoreactivity (arrow in E, immunohistochemistry using Texas red). The overlay of images D and E is shown in F. Bars: (A) 5 μm; (B and C) 1 μm; (D and F) 10 μm.
Figure 4.
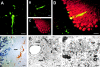
Characterization of GFP-expressing Purkinje cells in the brains of BM chimeras. 12–15 mo after transplantation of GFP-expressing BM cells, CNS engraftment of marrow-derived cells was evaluated in brain sections using direct fluorescence microscopy. In the cerebellum, single neurons located at the border of the granular and the molecular layer were found to express GFP in the perikaryon, axon, and dendrites (A). These cells were identified as Purkinje neurons by their expression of the calcium-binding protein, calbindin-D28K: section through a GFP-marked neuron (B, direct fluorescence microscopy) that shows calbindin immunoreactivity (arrow in C, immunohistochemistry using Texas red). The overlay (D) demonstrates that the two fluorophores are in the same cell. Immunohistochemical staining of semithin brain sections using an anti-GFP antibody visualized by diaminobenzidine revealed GFP immunoreactivity in Purkinje cell dendrites (E, arrow). Nuclei of adjacent granule cells and molecular layer interneurons were counterstained with methylene blue. Note that these cells did not show GFP immunoreactivity (E). Cytoplasmic GFP expression in Purkinje cell dendrites was demonstrated by immunoelectron microscopy for GFP (F and G). In F, a large GFP-labeled dendrite (indicated by arrows) is located in proximity to a blood vessel. Part of this dendrite is shown at higher magnification in G. The cytoplasm of the dendrite (D) contains mitochondria (M). Basket fibers terminate on the surface of the dendrite, and axons (Ax) of parallel fibers form asymmetric synaptic junctions with Purkinje cell dendritic thorns (arrows). Bars: (A–D) 50 μm; (E) 10 μm; (F) 1 μm; (G) 0.25 μm.
Figure 5.
Confocal microscopic analysis of BM-derived Purkinje cells. 12 mo after transplantation of BM cells from transgenic mice that ubiquitously express GFP, donor-derived Purkinje cells were visualized by confocal laser–scanning microscopy. The GFP fluorescence of an engrafted neuron (A) colocalized with calbindin immunoreactivity (B, immunohistochemistry using Texas red), characterizing the neuron as a Purkinje cell (C, overlay of images A and B). GFP-expressing Purkinje cells were also detected in chimeric mice 12 mo after transplantation of BM cells transduced with a GFP retroviral vector (D, maximum intensity projection of 16 consecutive scans). Another GFP-marked neuron is visible in the layer of GAD-expressing Purkinje cells (E, overlay of the maximum intensity projections of GFP and Texas red fluorescence visualizing GAD immunoreactivity). The single optical section through a GFP-positive Purkinje cell (F) revealed expression of GAD (G, immunohistochemistry using Texas red) in the Purkinje cell soma as well as in GABAergic nerve terminals (F, overlay of images F and G). Bars: (A–D) 25 μm; (E) 50 μm; (F–H) 25 μm.
Similar articles
- Bone marrow-derived cells expressing green fluorescent protein under the control of the glial fibrillary acidic protein promoter do not differentiate into astrocytes in vitro and in vivo.
Wehner T, Böntert M, Eyüpoglu I, Prass K, Prinz M, Klett FF, Heinze M, Bechmann I, Nitsch R, Kirchhoff F, Kettenmann H, Dirnagl U, Priller J. Wehner T, et al. J Neurosci. 2003 Jun 15;23(12):5004-11. doi: 10.1523/JNEUROSCI.23-12-05004.2003. J Neurosci. 2003. PMID: 12832523 Free PMC article. - Neuronal differentiation of murine bone marrow Thy-1- and Sca-1-positive cells.
Locatelli F, Corti S, Donadoni C, Guglieri M, Capra F, Strazzer S, Salani S, Del Bo R, Fortunato F, Bordoni A, Comi GP. Locatelli F, et al. J Hematother Stem Cell Res. 2003 Dec;12(6):727-34. doi: 10.1089/15258160360732740. J Hematother Stem Cell Res. 2003. PMID: 14977481 - Application of bone marrow-derived stem cells in experimental nephrology.
Ito T, Suzuki A, Okabe M, Imai E, Hori M. Ito T, et al. Exp Nephrol. 2001;9(6):444-50. doi: 10.1159/000052644. Exp Nephrol. 2001. PMID: 11702005 Review. - Binuclear Purkinje neurons.
Paltsyn AA, Komissarova SV. Paltsyn AA, et al. Patol Fiziol Eksp Ter. 2016 Oct-Dec;60(4):107-13. Patol Fiziol Eksp Ter. 2016. PMID: 29244931 Review.
Cited by
- Stem cell plasticity: the debate begins to clarify.
Spyridonidis A, Zeiser R, Follo M, Metaxas Y, Finke J. Spyridonidis A, et al. Stem Cell Rev. 2005;1(1):37-43. doi: 10.1385/scr:1:1:037. Stem Cell Rev. 2005. PMID: 17132873 Review. - Mesenchymal Stem Cells with Granulocyte Colony-Stimulating Factor Reduce Stress Oxidative Factors in Parkinson's Disease.
Ghahari L, Safari M, Rahimi Jaberi K, Jafari B, Safari K, Madadian M. Ghahari L, et al. Iran Biomed J. 2020 Mar;24(2):89-98. doi: 10.29252/ibj.24.2.89. Epub 2019 Nov 2. Iran Biomed J. 2020. PMID: 31677610 Free PMC article. - Regulatory Roles of Bone in Neurodegenerative Diseases.
Yu Z, Ling Z, Lu L, Zhao J, Chen X, Xu P, Zou X. Yu Z, et al. Front Aging Neurosci. 2020 Dec 21;12:610581. doi: 10.3389/fnagi.2020.610581. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33408628 Free PMC article. Review. - The nuclei of human adult stem cells can move within the cell and generate cellular protrusions to contact other cells.
Bueno C, García-Bernal D, Martínez S, Blanquer M, Moraleda JM. Bueno C, et al. Stem Cell Res Ther. 2024 Feb 7;15(1):32. doi: 10.1186/s13287-024-03638-y. Stem Cell Res Ther. 2024. PMID: 38321563 Free PMC article. - Increased cell fusion in cerebral cortex may contribute to poststroke regeneration.
Paltsyn A, Komissarova S, Dubrovin I, Kubatiev A. Paltsyn A, et al. Stroke Res Treat. 2013;2013:869327. doi: 10.1155/2013/869327. Epub 2013 Apr 3. Stroke Res Treat. 2013. PMID: 23691431 Free PMC article.
References
- Allay, J.A., D.A. Persons, J. Galipeau, J.M. Riberdy, R.A. Ashmun, R.L. Blakley, and B.P. Sorrentino. 1998. In vivo selection of retrovirally transduced hematopoietic stem cells. Nat. Med. 4:1136–1143. - PubMed
- Alvarez Otero, R., C. Sotelo, and R.-M. Alvarado-Mallart. 1993. Chick/quail chimeras with partial cerebellar grafts: an analysis of the origin and migration of cerebellar cells. J. Comp. Neurol. 333:597–615. - PubMed
- Anderson, W.A., and B.A. Flumerfeldt. 1984. Long-term effect of mossy fibre degeneration in the rat. J. Comp. Neurol. 227:414–423. - PubMed
- Bernocchi, G., S. Barni, and E. Scherini. 1986. The annual cycle of Erinaceus europaeus L. as a model for a further study of cytochemical heterogeneity in Purkinje neuron nuclei. Neuroscience. 17:427–437. - PubMed
- Bjornson, C.R.R., R.L. Rietze, B.A. Reynolds, M.C. Magli, and A.L. Vescovi. 1999. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 283:534–537. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous