Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors - PubMed (original) (raw)
Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors
R Sotillo et al. EMBO J. 2001.
Abstract
We have introduced a point mutation in the first coding exon of the locus encoding the cyclin-dependent kinase 4 (Cdk4) by homologous recombination in embryonic stem cells. This mutation (replacement of Arg24 by Cys) was first found in patients with hereditary melanoma and renders Cdk4 insensitive to INK4 inhibitors. Here, we report that primary embryonic fibroblasts expressing the mutant Cdk4R24C kinase are immortal and susceptible to transformation by Ras oncogenes. Moreover, homozygous Cdk4(R24C/R24C) mutant mice develop multiple tumors with almost complete penetrance. The most common neoplasia (endocrine tumors and hemangiosarcomas) are similar to those found in pRb(+/-) and p53(-/-) mice. This Cdk4 mutation cooperates with p53 and p27(Kip1) deficiencies in decreasing tumor latency and favoring development of specific tumor types. These results provide experimental evidence for a central role of Cdk4 regulation in cancer and provide a valuable model for testing the potential anti-tumor effect of Cdk4 inhibitors in vivo.
Figures
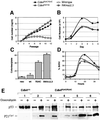
Fig. 1. Growth properties of Cdk4R24C/R24C MEFs. MEFs derived from wild-type (open circles), Cdk4R24C/R24C (filled circles), Cdk4neo/neo (open triangles) and INK4aΔ2,3 (gray squares) mice were utilized. (A) Growth of MEFs according to a 3T3 protocol; (B) cell density; (C) platting efficiency; (D) DNA synthesis after serum re-stimulation; and (E) functional analysis of the p53 pathway in immortal clones derived from wild-type MEFs (clone 1) or Cdk4R24C/R24C MEFs (clones 2–6). Immortal wild-type MEFs show overexpression of a p53 protein that is not able to induce p21_Cip1_ after treatment with doxorubycin. All five Cdk4R24C/R24C clones display normal p53 and p21_Cip1_ induction after doxorubycin treatment. Results shown in (A–D) correspond to an average of three to five different experiments.
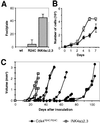
Fig. 2. Susceptibility of primary MEFs to transformation by Ras oncogenes. (A) Number of transformed foci per plate in wild-type (wt), Cdk4R24C/R24C or INK4aΔ2,3 MEFs after transfection with oncogenic H-Ras. (B) Growth curves of Ras-transformed clones derived from Cdk4R24C/R24C MEFS (filled circles) and INK4aΔ2,3 MEFs (squares). Data represent the average and standard deviation of two experiments with two different clones. (C) Growth of tumors after subcutaneous injection of individual Ras-transformed clones in nude mice. No tumors were observed after injection of non-transfected Cdk4R24C/R24C MEFs.
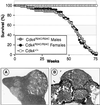
Fig. 3. (Top panel) Survival curve of Cdk4R24C/R24C male (gray circles) or female (black circles) mice compared with wild-type littermates (open circles). (A) Representative section of a hemangiosarcoma in the spleen (200×). (B) Metastasis of a hemangiosarcoma in the intestine epithelium (100×).
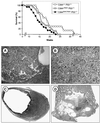
Fig. 4. Endocrine and epithelial non-endocrine tumors in Cdk4R24C/R24C mice. (A) Pancreatic endocrine tumor positive for insulin immunostaining (100×). (B–C) Adenomatous pancreatic islets showing cells positive for (B) insulin (blue) and glucagon (brown cells indicated by an arrow) or (C) insulin and PP (brown cells indicated by an arrow) (100×). (D) Leydig cell tumor with extension to the epididymis (200×). (E) Adenocarcinoma of the adenohypophysis immunoreactive for prolactin (200×). (F) Liver adenoma (200×). (G) Lung adenocarcinoma (100×). (H) Alveolar bronchiolar adenoma (400×). (I) Intestinal adenoma with in situ carcinoma (400×).
Fig. 5. (A) Cdk4 and (B) Cdk2 kinase activity in representative liver and testis samples from Cdk4R24C/R24C mice. Kinase activity was assayed after immunoprecipitation with Cdk4 or Cdk2 antibodies. R24C lanes depict examples of one out of four tissues in which we did not observe increased Cdk4 kinase activity and of two out of eight tissues in which the Cdk4 kinase activity was elevated. In none of the samples, we observed significantly increased Cdk2 activity. (C and D) Distribution of p27_Kip1_ in complexes formed with (C) Cdk4 or (D) Cdk2 in the above samples. IP, immunoprecipitation; WB, western blot. The migration of the Cdk4 and Cdk2 substrates, GST–pRB and Histone H1, respectively and of p27_Kip1_ is indicated by arrows.
Fig. 6. Cooperation between the Cdk4R24C allele and p53 deficiency. (Top panel) Tumor-free survival curve of p53-deficient mice carrying one or two Cdk4R24C alleles. (A–D) Representative sections of tumors obtained from Cdk4R24C/R24C;p53–/– double mutant mice. (A) Hemangiosarcoma located in the liver (100×). (B) Typical diffuse large cell lymphoma in spleen (400×). (C) Leiomyosarcoma with intermediate differentiation spreading to the large intestine/rectum area (200×). (D) Teratoma in the testis formed by mature structures such as cysts lined by columnar or epidermoid epithelium (center), bone (right) and cartilage (left), and a large immature area (200×).
Fig. 7. Cooperation between the Cdk4R24C mutation and loss of p27Kip1 in the development of pituitary tumors. (A) p27Kip1 expression in wild-type adenohypophysis. Most nuclei from endocrine cells are positive (1000×). (B) Cdk4R24C/R24C adenoma of the adenohypophysis showing areas of positive cells (arrows) and tumoral regions negative for p27Kip1 immunoreactivity (1000×). (C and D) Undifferentiated tumor of the pars intermedia in a Cdk4R24C/R24C;p27Kip1–/– mouse [hematoxylin and eosin staining; (C) 400×; (D)1000×]. (Right panel) Latency of pituitary tumors in animals derived from the crosses between Cdk4R24C/R24C and p27Kip1–/– mice. The _y_-axis represents the age of the mice at death in the different genotypes.
Similar articles
- Cooperation between Cdk4 and p27kip1 in tumor development: a preclinical model to evaluate cell cycle inhibitors with therapeutic activity.
Sotillo R, Renner O, Dubus P, Ruiz-Cabello J, Martín-Caballero J, Barbacid M, Carnero A, Malumbres M. Sotillo R, et al. Cancer Res. 2005 May 1;65(9):3846-52. doi: 10.1158/0008-5472.CAN-04-4195. Cancer Res. 2005. PMID: 15867383 - Genetic evidence for functional dependency of p18Ink4c on Cdk4.
Pei XH, Bai F, Tsutsui T, Kiyokawa H, Xiong Y. Pei XH, et al. Mol Cell Biol. 2004 Aug;24(15):6653-64. doi: 10.1128/MCB.24.15.6653-6664.2004. Mol Cell Biol. 2004. PMID: 15254233 Free PMC article. - Activating point mutations in cyclin-dependent kinase 4 are not seen in sporadic pituitary adenomas, insulinomas or Leydig cell tumours.
Vax VV, Bibi R, Diaz-Cano S, Gueorguiev M, Kola B, Borboli N, Bressac-de Paillerets B, Walker GJ, Dedov II, Grossman AB, Korbonits M. Vax VV, et al. J Endocrinol. 2003 Aug;178(2):301-10. doi: 10.1677/joe.0.1780301. J Endocrinol. 2003. PMID: 12904177 - Genes involved in melanoma: an overview of INK4a and other loci.
Castellano M, Parmiani G. Castellano M, et al. Melanoma Res. 1999 Oct;9(5):421-32. Melanoma Res. 1999. PMID: 10596908 Review. - Driving the cell cycle to cancer.
Malumbres M, Hunt SL, Sotillo R, Martín J, Odajima J, Martín A, Dubus P, Ortega S, Barbacid M. Malumbres M, et al. Adv Exp Med Biol. 2003;532:1-11. doi: 10.1007/978-1-4615-0081-0_1. Adv Exp Med Biol. 2003. PMID: 12908544 Review.
Cited by
- Therapeutic potential of CDK4/6 inhibitors in renal cell carcinoma.
Sager RA, Backe SJ, Ahanin E, Smith G, Nsouli I, Woodford MR, Bratslavsky G, Bourboulia D, Mollapour M. Sager RA, et al. Nat Rev Urol. 2022 May;19(5):305-320. doi: 10.1038/s41585-022-00571-8. Epub 2022 Mar 9. Nat Rev Urol. 2022. PMID: 35264774 Free PMC article. Review. - Cyclins and cyclin-dependent kinases: from biology to tumorigenesis and therapeutic opportunities.
Zabihi M, Lotfi R, Yousefi AM, Bashash D. Zabihi M, et al. J Cancer Res Clin Oncol. 2023 Apr;149(4):1585-1606. doi: 10.1007/s00432-022-04135-6. Epub 2022 Jul 4. J Cancer Res Clin Oncol. 2023. PMID: 35781526 Review. - Animal models of pituitary neoplasia.
Lines KE, Stevenson M, Thakker RV. Lines KE, et al. Mol Cell Endocrinol. 2016 Feb 5;421:68-81. doi: 10.1016/j.mce.2015.08.024. Epub 2015 Aug 28. Mol Cell Endocrinol. 2016. PMID: 26320859 Free PMC article. Review. - Determining the Balance Between Drug Efficacy and Safety by the Network and Biological System Profile of Its Therapeutic Target.
Li XX, Yin J, Tang J, Li Y, Yang Q, Xiao Z, Zhang R, Wang Y, Hong J, Tao L, Xue W, Zhu F. Li XX, et al. Front Pharmacol. 2018 Oct 31;9:1245. doi: 10.3389/fphar.2018.01245. eCollection 2018. Front Pharmacol. 2018. PMID: 30429792 Free PMC article. - In vivo proliferation of differentiated pancreatic islet beta cells in transgenic mice expressing mutated cyclin-dependent kinase 4.
Hino S, Yamaoka T, Yamashita Y, Yamada T, Hata J, Itakura M. Hino S, et al. Diabetologia. 2004 Oct;47(10):1819-30. doi: 10.1007/s00125-004-1522-4. Epub 2004 Oct 6. Diabetologia. 2004. PMID: 15480536
References
- Brooks G. and La Thangue,N.B. (1999) The cell cycle and drug discovery: the promise and the hope. Drug Discov. Today, 5, 455–464. - PubMed
- Corcoran M.M. et al. (1999) Disregulation of cyclin dependent kinase 6 expression in splenic marginal zone lymphoma through chromosome 7q translocations. Oncogene, 18, 6271–6277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous