p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis - PubMed (original) (raw)
p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis
G Rodier et al. EMBO J. 2001.
Abstract
The activity of the cyclin-dependent kinase inhibitor p27 is controlled by its concentration and subcellular localization. However, the mechanisms that regulate its intracellular transport are poorly understood. Here we show that p27 is phosphorylated on Ser10 in vivo and that mutation of Ser10 to Ala inhibits p27 cytoplasmic relocalization in response to mitogenic stimulation. In contrast, a fraction of wild-type p27 and a p27(S10D)-phospho-mimetic mutant translocates to the cytoplasm in the presence of mitogens. G1 nuclear export of p27 and its Ser10 phosphorylation precede cyclin-dependent kinase 2 (Cdk2) activation and degradation of the bulk of p27. Interestingly, leptomycin B-mediated nuclear accumulation accelerates the turnover of endogenous p27; the p27(S10A) mutant, which is trapped in the nucleus, has a shorter half-life than wild-type p27 and the p27(S10D) mutant. In summary, p27 is efficiently degraded in the nucleus and phosphorylation of Ser10 is necessary for the nuclear to cytoplasmic redistribution of a fraction of p27 in response to mitogenic stimulation. This cytoplasmic localization may serve to decrease the abundance of p27 in the nucleus below a certain threshold required for activation of cyclin-Cdk2 complexes.
Figures
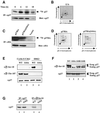
Fig. 1. p27 is phosphorylated on Ser10 in vivo. (A) Quiescent Rat1 cells were labeled with 0.5 mCi/ml [32P]phosphoric acid for 4 h and then stimulated with 10% serum for the times indicated. The cells were lysed and the endogenous p27 was immunoprecipitated with anti-p27 antibody. The precipitated proteins were resolved by SDS–gel electrophoresis, transferred to PVDF membrane and analyzed by autoradiography (top). The abundance of p27 protein was monitored by immunoblotting (bottom). (B) The 32P-labeled p27 protein band (from cells stimulated for 12 h with serum) was excised from the membrane and digested with trypsin. The resulting phosphopeptides were separated by thin layer electrophoresis followed by ascending chromatography. The arrow denotes the position of sample application. (C) Rat1 cells were transfected with HA-tagged wild-type p27 or p27(S10A) mutant. After 48 h, the cells were labeled with [32P]phosphoric acid and ectopic p27 was immunoprecipitated from cellular lysates with an anti-HA antibody. The extent of phosphorylation and abundance of ectopic p27 were analyzed as in (A). (D) Phosphopeptide mapping analysis of ectopically expressed HA-tagged p27 and p27(S10A) mutant. (E) Specificity of p27 phospho-Ser10 antibody. Recombinant purified wild-type p27 and p27(S10A) mutant were phosphorylated in vitro by either cyclin E–Cdk2 (lanes 1 and 2) or ERK2 (lanes 3 and 4). Phosphorylation products were analyzed by immunoblotting with anti-phospho-Ser10-specific antibody (top), anti-phospho-Thr187 specific antibody (middle) or with a mouse anti-p27 antibody (bottom). (F) NIH 3T3 cells were transfected with human wild-type p27 (lane 1), p27(S10A; lane 2), p27(S10D; lane 3) or p27(S10E; lane 4) mutants. Protein extracts were analyzed by immunoblotting with either anti-phospho-Ser10 antibody or anti-p27 antibody. The human p27 can be distinguished from the endogenous mouse p27 because it migrates slower on SDS gels. (G) Lysates from NIH 3T3 cells transfected with wild-type p27 (wt) or p27(S10A) mutant were subjected to immunoprecipitation with either anti-p27 antibody (lanes 1 and 2) or anti-phospho-Ser10 antibody (lanes 3 and 4). The precipitated proteins were analyzed by immunoblotting with anti-p27 antibody.
Fig. 2. Phosphorylation of p27 on Ser10 is cell cycle regulated. (A) Mouse T lymphocytes were stimulated with concanavalin A and interleukin-2 for the times indicated. Cellular extracts were analyzed by immunoblotting with specific antibodies to the indicated proteins. (B) NIH 3T3 fibroblasts were synchronized in G0/G1 by serum starvation and restimulated with 10% serum for the times indicated. Cell extracts were prepared and incubated with recombinant purified p27 in kinase assay buffer as described in Materials and methods. The reaction products were analyzed by immunoblotting using anti-phospho-Ser10- (upper) and Thr187 (lower)-specific antibodies. (C) MCF-7 breast cancer cells were depleted of estradiol and treated with 1 µM tamoxifen for the times indicated (lanes 1–4). After 48 h, arrested cells were stimulated to re-enter the cell cycle by addition of 500 nM estradiol for the intervals of time indicated (lanes 5–9). Cell extracts were analyzed by immunoblotting with specific antibodies to the indicated proteins. (D) Cell extracts (50 µg protein) from proliferating (AS) or tamoxifen-treated (TMX) MCF-7 cells were sequentially immunoprecipitated four times with anti-phospho-Ser10 antibody. The resultant pellets (lanes 1–4 and 6–9) and the final supernatant (lanes 5 and 10), as well as the input lysate (lanes 11 and 12), were analyzed by immunoblotting with anti-p27 antibody. (E) MCF-7 cell extracts described in (C) (lanes 1–4) were subjected to immunoprecipitation with anti-p27 antibody and analyzed by immunoblotting with specific antibodies to the indicated proteins. (F) The same MCF-7 cell extracts described in (E) were subjected to immunoprecipitation with anti-phospho-Ser10 antibody and analyzed by immunoblotting as indicated.
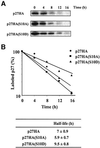
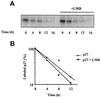
Fig. 3. Phosphorylation of p27 on Ser10 increases its half-life. (A) Rat1 cells were transfected with HA-tagged p27 wild-type or p27(Ser10) mutants and serum deprived for 24 h. The cells were then pulse-labeled for 2 h with [35S]methionine or [35S]cysteine and chased for the times indicated in fresh medium containing 10% serum. Cell lysates were subjected to immunoprecipitation with anti-HA antibody and the labeled p27 protein was analyzed by SDS–gel electrophoresis and fluorography. (B) Densitometric analysis of p27 degradation rate. Data points correspond to the experiment shown in (A). The half-life values of wild-type and mutant p27 represent the mean ± SEM of four independent pulse–chase experiments.
Fig. 4. Phosphorylation of p27 on Ser10 does not affect its interaction with the F-box protein Skp2. Rat1 cells were transfected with HA-tagged p27 wild-type or p27 mutants and treated for 5 h with 5 µM MG132 (to increase the amount of p27 phosphorylated on Thr187). Lysates were prepared, incubated with in vitro translated [35S]Myc6Skp2 for 3 h and subjected to immunoprecipitation with anti-HA antibody. The precipitated proteins were resolved by SDS–gel electrophoresis, transferred to nitrocellulose membrane and visualized by autoradiography (upper). The membrane was further subjected to immunoblot analysis with antibodies to p27 (middle) and phospho-Thr187 (lower).
Fig. 5. Phosphorylation on Ser10 does not affect p27 in vitro ubiquitylation. 35S-labeled in vitro translated p27 wild-type (lanes 1–5), p27(S10A) mutant (lanes 6–10) or p27(S10D) mutant (lanes 11–15) were subjected to a ubiquitylation reaction for the times indicated using purified components as described in Materials and methods. The reaction products were analyzed by SDS–gel electrophoresis followed by autoradiography. The bracket on the left side marks a ladder of bands corresponding to polyubiquitylated p27.
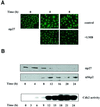
Fig. 6. Mitogenic stimulation promotes the nuclear export of a fraction of p27. (A) Rat1 cells were made quiescent by serum starvation and restimulated to enter the cell cycle by addition of 10% serum for the times indicated. The subcellular localization of endogenous p27 was determined by fluorescence microscopy. When present, LMB (2 ng/ml) was added simultaneously with serum. (B) Rat1 cells were made quiescent and restimulated with serum for the times indicated. The expression of p27 and Skp2 was monitored by immunoblotting using specific antibodies. The activity of Cdk2 was assayed using histone H1 as substrate.
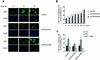
Fig. 7. Phosphorylation of Ser10 is necessary for the nuclear to cytoplasmic translocation of p27 upon cell cycle re-entry. (A) Rat1 cells were transfected with HA-tagged p27 wild-type or Ser10 mutants and serum starved for 24 h. The cells were then stimulated with 10% serum for the times indicated. The subcellular localization of ectopic p27 was determined by fluorescence microscopy after staining with anti-HA antibody. (B) Quantitative evaluation of the cellular localization of p27 wild-type and Ser10 mutants. At least 300 cells were scored for each coverslip. The results are expressed as the percentage of cells showing both nuclear and cytoplasmic staining. The graph represents the mean ± SEM of three separate experiments. (C) Effect of LMB. Rat1 cells were transfected with HA-tagged p27 wild-type or Ser10 mutants. After 24 h, the cells were made quiescent and restimulated with 10% serum for 24 h in the absence or presence of 2 ng/ml LMB. The results are expressed as the percentage of cells showing both nuclear and cytoplasmic staining. The graph represents the mean ± SEM of five separate experiments.
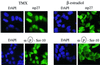
Fig. 8. p27 phosphorylated on Ser10 is localized into the cytoplasm of proliferating cells. MCF-7 cells were growth arrested by depletion of estradiol and treatment with tamoxifen for 48 h. The cells were then restimulated to enter the cell cycle by addition of 500 nM estradiol for a period of 8 h. The subcellular localizations of endogenous p27 and Ser10 phosphorylated p27 were determined by immunofluorescence using anti-p27 and anti-phospho-Ser10, respectively. Exposure time in proliferating cells was enhanced to better visualize cytoplasmic p27.
Fig. 9. Effect of LMB on the degradation rate of endogenous p27. (A) Rat1 cells were made quiescent by serum starvation for 24 h. Pulse–chase experiments were performed as described in the legend to Figure 3 except that LMB was added during the chase period. The endogenous p27 protein was immunoprecipitated with anti-p27 and analyzed by SDS–gel electrophoresis and fluorography. (B) Densitometric analysis of p27 degradation rate.
Similar articles
- Estrogens down-regulate p27Kip1 in breast cancer cells through Skp2 and through nuclear export mediated by the ERK pathway.
Foster JS, Fernando RI, Ishida N, Nakayama KI, Wimalasena J. Foster JS, et al. J Biol Chem. 2003 Oct 17;278(42):41355-66. doi: 10.1074/jbc.M302830200. Epub 2003 Aug 6. J Biol Chem. 2003. PMID: 12904306 - Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export.
Ishida N, Hara T, Kamura T, Yoshida M, Nakayama K, Nakayama KI. Ishida N, et al. J Biol Chem. 2002 Apr 26;277(17):14355-8. doi: 10.1074/jbc.C100762200. Epub 2002 Mar 11. J Biol Chem. 2002. PMID: 11889117 - Mutations of phosphorylation sites Ser10 and Thr187 of p27Kip1 abolish cytoplasmic redistribution but do not abrogate G0/1 phase arrest in the HepG2 cell line.
Guan X, Chen L, Wang J, Geng H, Chu X, Zhang Q, Du L, De W. Guan X, et al. Biochem Biophys Res Commun. 2006 Sep 1;347(3):601-7. doi: 10.1016/j.bbrc.2006.06.114. Epub 2006 Jun 28. Biochem Biophys Res Commun. 2006. PMID: 16842750 - Role of serine 10 phosphorylation in p27 stabilization revealed by analysis of p27 knock-in mice harboring a serine 10 mutation.
Kotake Y, Nakayama K, Ishida N, Nakayama KI. Kotake Y, et al. J Biol Chem. 2005 Jan 14;280(2):1095-102. doi: 10.1074/jbc.M406117200. Epub 2004 Nov 3. J Biol Chem. 2005. PMID: 15528185 - Cytoplasmic displacement of cyclin E-cdk2 inhibitors p21Cip1 and p27Kip1 in anchorage-independent cells.
Orend G, Hunter T, Ruoslahti E. Orend G, et al. Oncogene. 1998 May;16(20):2575-83. doi: 10.1038/sj.onc.1201791. Oncogene. 1998. PMID: 9632134
Cited by
- Casein kinase iγ2 impairs fibroblasts actin stress fibers formation and delays cell cycle progression in g1.
Latreille M, Abu-Thuraia A, Oliva R, Zuo D, Larose L. Latreille M, et al. Int J Cell Biol. 2012;2012:684684. doi: 10.1155/2012/684684. Epub 2012 Mar 7. Int J Cell Biol. 2012. PMID: 22496693 Free PMC article. - The CRM1 nuclear export protein in normal development and disease.
Nguyen KT, Holloway MP, Altura RA. Nguyen KT, et al. Int J Biochem Mol Biol. 2012;3(2):137-51. Epub 2012 May 18. Int J Biochem Mol Biol. 2012. PMID: 22773955 Free PMC article. - C-terminal phosphorylation controls the stability and function of p27kip1.
Kossatz U, Vervoorts J, Nickeleit I, Sundberg HA, Arthur JS, Manns MP, Malek NP. Kossatz U, et al. EMBO J. 2006 Nov 1;25(21):5159-70. doi: 10.1038/sj.emboj.7601388. Epub 2006 Oct 19. EMBO J. 2006. PMID: 17053782 Free PMC article. - Phosphorylation of p27Kip1 at Thr187 by cyclin-dependent kinase 5 modulates neural stem cell differentiation.
Zheng YL, Li BS, Rudrabhatla P, Shukla V, Amin ND, Maric D, Kesavapany S, Kanungo J, Pareek TK, Takahashi S, Grant P, Kulkarni AB, Pant HC. Zheng YL, et al. Mol Biol Cell. 2010 Oct 15;21(20):3601-14. doi: 10.1091/mbc.E10-01-0054. Epub 2010 Sep 1. Mol Biol Cell. 2010. PMID: 20810788 Free PMC article. - Adhesion to fibronectin induces p27(Kip1) nuclear accumulation through down-regulation of Jab1 and contributes to cell adhesion-mediated drug resistance (CAM-DR) in RPMI 8,226 cells.
Fei M, Hang Q, Hou S, He S, Ruan C. Fei M, et al. Mol Cell Biochem. 2014 Jan;386(1-2):177-87. doi: 10.1007/s11010-013-1856-7. Epub 2013 Oct 30. Mol Cell Biochem. 2014. PMID: 24170542
References
- Boussiotis V.A., Freeman,G.J., Taylor,P.A., Berezovskaya,A., Grass,I., Blazar,B.R. and Nadler,L.M. (2000) p27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nature Med., 6, 290–297. - PubMed
- Carrano A.C., Eytan,E., Hershko,A. and Pagano,M. (1999) SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biol., 1, 193–199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases