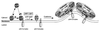Recruitment to Golgi membranes of ADP-ribosylation factor 1 is mediated by the cytoplasmic domain of p23 - PubMed (original) (raw)
Recruitment to Golgi membranes of ADP-ribosylation factor 1 is mediated by the cytoplasmic domain of p23
D U Gommel et al. EMBO J. 2001.
Abstract
Binding to Golgi membranes of ADP ribosylation factor 1 (ARF1) is the first event in the initiation of COPI coat assembly. Based on binding studies, a proteinaceous receptor has been proposed to be critical for this process. We now report that p23, a member of the p24 family of Golgi-resident transmembrane proteins, is involved in ARF1 binding to membranes. Using a cross-link approach based on a photolabile peptide corresponding to the cytoplasmic domain of p23, the GDP form of ARF1 (ARF1-GDP) is shown to interact with p23 whereas ARF1-GTP has no detectable affinity to p23. The p23 binding is shown to localize specifically to a 22 amino acid C-terminal fragment of ARF1. While a monomeric form of a non-photolabile p23 peptide does not significantly inhibit formation of the cross-link product, the corresponding dimeric form does compete efficiently for this interaction. Consistently, the dimeric p23 peptide strongly inhibits ARF1 binding to native Golgi membranes suggesting that an oligomeric form of p23 acts as a receptor for ARF1 before nucleotide exchange takes place.
Figures
Fig. 1. Direct interaction of ARF1-GDP with the cytoplasmic domain of p23. (A) Nucleotide-dependent photo-cross-linking between a photolabile peptide corresponding to the cytoplasmic domain of p23 (50 µM p23-CT*; see Table I) with 2.5 µM of either recombinant myristoylated ARF1 (mARF1) or recombinant NΔ17ARF1 was conducted in the presence of 3 mM l-α-dimyristoyl-phosphatidyl-choline liposomes. Components were mixed as indicated in a total volume of 20 µl. Control conditions included omission of UV-irradiation (lane 1), omission of p23-CT* (lane 2) or omission of recombinant ARF1 (lane 6). In lanes 4 and 5, mARF1 or NΔ17ARF1 were pre-incubated with the nucleotide exchange factor ARNO (0.8 µM) in the presence of 50 µM GDPβS or GTPγS for 30 min at 37°C. Incorporation of GTP was monitored by the addition of trace amounts of [α-32P]GTP and found to be complete at 70 and 95% for mARF1 and NΔ17ARF1, respectively (data not shown). Proteins in lane 3 were pre-incubated with GDPβS in the absence of ARNO. Photo-cross-link reactions were conducted as described under Materials and methods and analyzed on 16.5 % tricine SDS gels (Schägger and von Jagow, 1987) followed by western blotting and immunodetection with antibodies against ARF1 (αARF1) and p23-CT (αp23-CT). (B) NΔ17ARF1 was incubated with p23-CT* as described in (A) in a final volume of 60 µl. The sample was split into three aliquots. One aliquot was irradiated (lane 1) and the two other aliquots were incubated with ARNO (0.8 µM), either in the presence of 50 µM GTPγS (lane 2) or GDPβS (lane 3). Following irradiation, the samples were analyzed as described above.
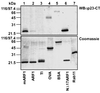
Fig. 2. p23-CT* interacts specifically with mARF1. Comparable amounts (∼1 µg) of mARF1, ARF1, TI, ovalbumin (OVA), BSA, NΔ17ARF1 and rab11 were incubated in the absence of liposomes with the photolabile peptide p23-CT* (50 µM; see Table I) and irradiated as described in Materials and methods. Samples were analyzed on 16.5% tricine SDS gels (Schägger and von Jagow, 1987) and were further processed for either western blotting and immunodetection against p23-CT (WBαp23-CT, top) or Coomassie Blue staining (bottom).
Fig. 3. An oligomeric form of p23-CT* interacts with ARF1-GDP.(A) Competition experiments were performed by incubating 12.5 µM p23-CT* (see Table I) and 2.5 µM mARF1 in the presence of excess amounts of wild-type p23-CT, the cytoplasmic domain of Wbp1 (Wbp1-CT) or the cytoplasmic domain of the KDEL receptor (KDEL-R-CT) in the absence of liposomes. See Table I for sequence information. Both monomeric and preformed dimers were used at the concentrations indicated. mARF1 was pre-incubated with candidate competitor peptides for 30 min at 25°C. Photo-cross-link reactions were conducted as described in Materials and methods. Cross-link products were analyzed on 16.5% tricine SDS gels (Schägger and von Jagow, 1987) followed by western blotting and immunodetection with an antibody directed against p23-CT. (B) Photo-cross-linking between p23-CT* and mARF1 was analyzed after prolonged gel electrophoresis as described above.
Fig. 4. Interface between NΔ17ARF1 and p23-CT*. (A) 25 nmol NΔ17ARF1 was incubated with 50 nmol of p23-CT* in the presence of GDPβS and irradiated as described in Materials and methods. Samples of starting material and cross-linked NΔ17ARF1 were separated by SDS–PAGE with subsequent Coomassie Blue staining (lanes 1 and 2). Bands # and + were dissected from various lanes of an SDS gel and cleaved with CNBr as described in Materials and methods. Cleavage products were analyzed by SDS–PAGE and the band positive for a cross-link product (band °) was analyzed by Edman microsequencing. (B) NΔ17ARF1 was cleaved with CNBr, purified and analyzed by MALDI-TOF as described in Materials and methods. The peak at 5355.5 Da corresponds to the molecular mass of residues 135–181 of NΔ17ARF1. (C) NΔ17ARF1 was cross-linked to p23-CT*, cleaved with CNBr, purified and analyzed by MALDI-TOF as decribed in Materials and methods. The molecular mass indicated by ° corresponds to the cross-linked C-terminal fragment (residues 135–181) of NΔ17ARF1.
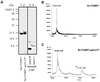
Fig. 5. Interface between NΔ17ARF1 and p23-CT*. (A) Samples of 8.3 nmol of NΔ17ARF1, either mock-treated (lanes 1, 3, 5 and 7) or cross-linked to p23-CT* (lanes 2, 4, 6 and 8), were cleaved with NTCB as described in Materials and methods. Samples of starting material (lanes 1, 2, 5 and 6) and NTCB-cleaved material (lanes 3, 4, 7 and 8) were separated by SDS–PAGE with subsequent western blotting using antibodies directed against ARF1 (lanes 1–4) or p23-CT (lanes 5–8). (B) NΔ17ARF1 was cleaved with NTCB, purified and analyzed by MALDI-TOF as decribed in Materials and methods. The peak at 2593.4 Da corresponds to the molecular mass of residues 159–181 of NΔ17ARF1, while 5185.5 Da represents a dimer of this fragment. (C) NΔ17ARF1 was cross-linked to p23-CT*, cleaved with NTCB, purified and analyzed by MALDI-TOF as decribed in Materials and methods. The molecular mass indicated by an asterisk corresponds to the cross-linked C-terminal fragment (residues 159–181) of NΔ17ARF1. The peak at 1809.6 Da corresponds to the molecular mass of p23-CT* cross-linked to H2O, while 5187.0 Da represents a dimer of the C-terminal fragment.
Fig. 6. Dimeric p23-CT inhibits recruitment to Golgi membranes of ARF1-GDP. Binding studies were performed in a final volume of 50 µl using mARF1 (0.4 µM) and rat liver Golgi membranes (9.0 µg) in the presence of excess amounts of peptides corresponding to the cytoplasmic domains of mammalian p24 proteins as well as control proteins (p23-CT, p24-CT, p25-CT, p26-CT, p27-CT, Wbp1-CT and KDEL-R-CT; for sequence information see Table I). Incubations contained either GTPγS or GDPβS as indicated. Total ARF1 binding to membranes was typically enhanced 3- to 5-fold in the presence of GTPγS (data not shown). In the presence of GDPβS, the amount of mARF1 was increased (0.8 µM) and exposition times were elongated in order to obtain signal intensities comparable to ARF1 binding experiments in the presence of GTPγS. For additional details see Materials and methods. (A) GTPγS- and GDPβS-dependent mARF1 binding was analyzed in the presence of monomeric (120 µM) and dimeric (60 µM) peptides, respectively. After incubation the membranes were collected by centrifugation through a 15% (w/v) sucrose cushion followed by analysis of the membrane pellet employing SDS–PAGE and western blotting for immunodetection of ARF1 (αARF1; Palmer et al., 1993). In order to normalize for recovery of Golgi membranes, each experimental condition was analyzed for the amount of endogenous p23 as a Golgi marker utilizing an antibody directed against the lumenal part of p23 (αp23-lum; Sohn et al., 1996). (B) Concentration dependence of competition in the presence of either GDPβS or GTPγS. Peptides were added at the concentrations indicated. Determination of membrane-bound ARF1 was performed as described in (A).
Fig. 7. A model for the recruitment to Golgi membranes of ARF1. Soluble ARF1-GDP binds to membrane phospholipids at low affinity. Upon binding to a p23 oligomer this interaction is stabilized. If, subsequently, a nucleotide exchange factor acts on ARF1-GDP, the resulting ARF1-GTP is released from p23 and two binding sites for coatomer are generated in close proximity: membrane-bound ARF1-GTP and a p23 oligomer. For further details, see Discussion.
Similar articles
- Multiple and stepwise interactions between coatomer and ADP-ribosylation factor-1 (Arf1)-GTP.
Sun Z, Anderl F, Fröhlich K, Zhao L, Hanke S, Brügger B, Wieland F, Béthune J. Sun Z, et al. Traffic. 2007 May;8(5):582-93. doi: 10.1111/j.1600-0854.2007.00554.x. Traffic. 2007. PMID: 17451557 - Correct targeting of plant ARF GTPases relies on distinct protein domains.
Matheson LA, Suri SS, Hanton SL, Chatre L, Brandizzi F. Matheson LA, et al. Traffic. 2008 Jan;9(1):103-20. doi: 10.1111/j.1600-0854.2007.00671.x. Epub 2007 Nov 27. Traffic. 2008. PMID: 17988226 - Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport.
Presley JF, Ward TH, Pfeifer AC, Siggia ED, Phair RD, Lippincott-Schwartz J. Presley JF, et al. Nature. 2002 May 9;417(6885):187-93. doi: 10.1038/417187a. Nature. 2002. PMID: 12000962 - The COPI system: molecular mechanisms and function.
Beck R, Rawet M, Wieland FT, Cassel D. Beck R, et al. FEBS Lett. 2009 Sep 3;583(17):2701-9. doi: 10.1016/j.febslet.2009.07.032. Epub 2009 Jul 22. FEBS Lett. 2009. PMID: 19631211 Review. - Membrane curvature and the control of GTP hydrolysis in Arf1 during COPI vesicle formation.
Antonny B, Bigay J, Casella JF, Drin G, Mesmin B, Gounon P. Antonny B, et al. Biochem Soc Trans. 2005 Aug;33(Pt 4):619-22. doi: 10.1042/BST0330619. Biochem Soc Trans. 2005. PMID: 16042557 Review.
Cited by
- Following the fate in vivo of COPI vesicles generated in vitro.
Rutz C, Satoh A, Ronchi P, Brügger B, Warren G, Wieland FT. Rutz C, et al. Traffic. 2009 Aug;10(8):994-1005. doi: 10.1111/j.1600-0854.2009.00934.x. Epub 2009 Apr 25. Traffic. 2009. PMID: 19497049 Free PMC article. - Role of adaptor proteins in secretory granule biogenesis and maturation.
Bonnemaison ML, Eipper BA, Mains RE. Bonnemaison ML, et al. Front Endocrinol (Lausanne). 2013 Aug 14;4:101. doi: 10.3389/fendo.2013.00101. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23966980 Free PMC article. - A novel physiological role for ARF1 in the formation of bidirectional tubules from the Golgi.
Bottanelli F, Kilian N, Ernst AM, Rivera-Molina F, Schroeder LK, Kromann EB, Lessard MD, Erdmann RS, Schepartz A, Baddeley D, Bewersdorf J, Toomre D, Rothman JE. Bottanelli F, et al. Mol Biol Cell. 2017 Jun 15;28(12):1676-1687. doi: 10.1091/mbc.E16-12-0863. Epub 2017 Apr 20. Mol Biol Cell. 2017. PMID: 28428254 Free PMC article. - Coatomer, the coat protein of COPI transport vesicles, discriminates endoplasmic reticulum residents from p24 proteins.
Béthune J, Kol M, Hoffmann J, Reckmann I, Brügger B, Wieland F. Béthune J, et al. Mol Cell Biol. 2006 Nov;26(21):8011-21. doi: 10.1128/MCB.01055-06. Epub 2006 Aug 28. Mol Cell Biol. 2006. PMID: 16940185 Free PMC article. - The ArfGAP Glo3 is required for the generation of COPI vesicles.
Lewis SM, Poon PP, Singer RA, Johnston GC, Spang A. Lewis SM, et al. Mol Biol Cell. 2004 Sep;15(9):4064-72. doi: 10.1091/mbc.e04-04-0316. Epub 2004 Jul 14. Mol Biol Cell. 2004. PMID: 15254269 Free PMC article.
References
- Amor J.C., Harrison,D.H., Kahn,R.A. and Ringe,D. (1994) Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature, 372, 704–708. - PubMed
- Antonny B., Beraud-Dufour,S., Chardin,P. and Chabre,M. (1997) N-terminal hydrophobic residues of the G protein ADP ribosylation factor 1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry, 36, 4675–4684. - PubMed
- Belden W.J. and Barlowe,C. (1996) Erv25p, a component of COPII-coated vesicles, forms a complex with Emp24p that is required for efficient endoplasmic reticulum to Golgi transport. J. Biol. Chem., 271, 26939–26949. - PubMed
- Beraud-Dufour S., Paris,S., Chabre,M. and Antonny,B. (1999) Dual interaction of ADP ribosylation factor 1 with Sec7 domain and with lipid membranes during catalysis of guanine nucleotide exchange. J. Biol. Chem., 274, 37629–37636. - PubMed
- Bremser M., Nickel,W., Schweikert,M., Ravazzola,M., Amherdt,M., Hughes,C.A., Söllner,T.H., Rothman,J.E. and Wieland,F.T. (1999) Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell, 96, 495–506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases