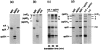A dominant interference collagen X mutation disrupts hypertrophic chondrocyte pericellular matrix and glycosaminoglycan and proteoglycan distribution in transgenic mice - PubMed (original) (raw)
A dominant interference collagen X mutation disrupts hypertrophic chondrocyte pericellular matrix and glycosaminoglycan and proteoglycan distribution in transgenic mice
O Jacenko et al. Am J Pathol. 2001 Dec.
Abstract
Collagen X transgenic (Tg) mice displayed skeleto-hematopoietic defects in tissues derived by endochondral skeletogenesis.(1) Here we demonstrate that co-expression of the transgene product containing truncated chicken collagen X with full-length mouse collagen X in a cell-free translation system yielded chicken-mouse hybrid trimers and truncated chicken homotrimers; this indicated that the mutant could assemble with endogenous collagen X and thus had potential for dominant interference. Moreover, species-specific collagen X antibodies co-localized the transgene product with endogenous collagen X to hypertrophic cartilage in growth plates and ossification centers; proliferative chondrocytes also stained diffusely. Electron microscopy revealed a disrupted hexagonal lattice network in the hypertrophic chondrocyte pericellular matrix in Tg growth plates, as well as altered mineral deposition. Ruthenium hexamine trichloride-positive aggregates, likely glycosaminoglycans (GAGs)/proteoglycans (PGs), were also dispersed throughout the chondro-osseous junction. These defects likely resulted from transgene co-localization and dominant interference with endogenous collagen X. Moreover, altered GAG/PG distribution in growth plates of both collagen X Tg and null mice was confirmed by a paucity of staining for hyaluronan and heparan sulfate PG. A provocative hypothesis links the disruption of the collagen X pericellular network and GAG/PG decompartmentalization to the potential locus for hematopoietic failure in the collagen X mice.
Figures
Figure 1.
Co-expression of truncated chicken with full-length mouse collagen X chains in a cell-free translation system promotes formation of chicken and mouse homotrimers and heterotrimers. Chicken collagen X cDNAs including SpLX (21 codon deletion) and SpLXH (293-codon deletion), and mX, the full-length mouse collagen X cDNA, were transcribed and translated in a coupled cell-free translation system. Resultant [35S]-methionine-labeled products were analyzed by 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. a: Standard cell-free translation. Translation products from plasmids SpLX, SpLXH, and mX correspond to monomers. b: Cell-free translation under conditions favoring associations. Same samples as in a. (SpLX)3, (SpLX)3, and (mX)3 represent homotrimeric products. c: Co-translation of chicken and mouse collagen X plasmids under conditions favoring homotrimer and heterotrimer production. Trimer formation is shown as a function of translation time. Bands a and b represent the heterotrimers (mX)2(SpLXH) (band a) and (mX)(SpLXH)2 (band b). Note that with increasing translation time, stoichiometry between products remains constant. d: Cell-free co-translation of chicken and mouse collagen X plasmids in the presence of microsomal membranes. Monomers and homotrimers are as in a and b. Bands a–d represent the heterotrimers (SpLX2(SpLXH) (band a), (SpLX)(SpLXH)2 (band b), (mX)2(SpLXH) (band c), and (mX)(SpLXH)2 (band d).
Figure 2.
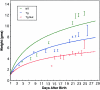
Growth retardation in collagen X Tg mice. Tg mice were indistinguishable from wild-type (WT) controls at birth, but exhibited a range of defects by week 3. Variable dwarfism in ∼75% of the mice (Tg), or perinatal lethality in approximately 25% of the mice (Mut), could be detected as reduced weight. For this study, all Tg and control littermates were weighed at specified time points, and then grouped based on whether or not they developed perinatal lethality. The best-fit line program was used to generate plot.
Figure 3.
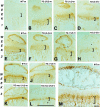
Co-localization of transgene product with endogenous collagen X. Longitudinal tibial sections from wild-type (Wt) control, collagen X Tg (Tg), and null (KO) mice at birth, and of embryonic day 18 chick sterna (St) were either stained with H&E (A), or with antibodies against chicken (B–D) or mouse (E–H) collagen X. Note lack of cross-reactivity between chicken antibodies and mouse proteins (B), but staining of hypertrophic cartilage in Tg growth plates (C) and chick sterna (D). Mouse antibodies similarly stain hypertrophic cartilage in both WT (F) and Tg (G) mice, but do not cross-react with noncollagen X mouse proteins (E), or with chick hypertrophic cartilage (H). Faint collagen X reactivity extends into proliferative cartilage with both antibodies (C, D, F, and G). Brackets approximate width of hypertrophic cartilage. Scale bars, 100 μm.
Figure 4.
Temporal co-expression of mouse collagen X and transgene product. Longitudinal tibial sections at weeks 1 (A–D), 2 (E–H), and 3 (I–M) from wild-type control (WT; A, E, I, and M), and collagen X Tg mice from lines 3-2 [Tg (3-2) construct 1600-392 containing the 1.6-kb promotor and SpLXH cDNA with the 293-amino acid triple-helical deletion; B, D, F, H, K, and L] and 1-2 [Tg (1-2) construct 4200-21 containing the 4.2-kb promotor and SpLX cDNA with the 21-amino acid triple-helical deletion; G and J]; K and L: perinatal-lethal mutants (Tg-mut). Sections are stained with antibodies against mouse (m; B, C, E–G, I–K, and M) or chicken (c; D, H, and L) collagen X; controls include omission of primary antibodies (A). Mouse collagen X and transgene product co-localize to hypertrophic cartilage at week 1, with faint staining also in proliferative cartilage (A–D). By week 2, antibody reactivity involves hypertrophic chondrocytes within secondary ossification centers; adjacent resting cartilage (E–H, arrows) is negative. At week 3 (I–M), antibody reactivity is strongest in hypertrophic chondrocytes around secondary ossification centers, and within growth plates where it concentrates to a strip of less mature hypertrophic cells (M). Decreased trabecular bone and growth plate compressions are most pronounced in perinatal lethal mutants (K and L). Brackets approximate width of hypertrophic cartilage. Scale bars: 1 mm (A–L); 100 μm (M).
Figure 5.
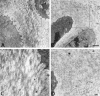
Ultrastructure of hypertrophic cartilage of week 3 tibial growth plates reveals a pericellular matrix defect in collagen X Tg mice. The hypertrophic chondrocyte cell layer before the terminal layer abutting the trabecular bone and marrow is depicted. In controls, hypertrophic chondrocytes were surrounded by a gray zone corresponding to the pericellular matrix that consisted of a fine meshwork (A, arrows in boxed-in region); this matrix was reduced or lacking in the Tg mice (B, arrow). Higher magnification revealed a lattice-like array in the pericellular matrix of hypertrophic chondrocytes from controls (C). In Tg mice, no ordered networks were evident; instead, RHT-positive aggregates accumulated near cell surfaces (D). Scale bars: 2 μm (A and B); 200 nm (C and D).
Figure 6.
Ultrastructure of hypertrophic cartilage of week 3 tibial growth plates reveals altered mineral deposition in collagen X Tg mice. The hypertrophic chondrocyte cell layer before the terminal layer abutting the trabecular bone and marrow is depicted. Occasional mineral deposits were observed adjacent to the hypertrophic chondrocyte surface in Tg mice (B, arrow), whereas these regions in controls remained mineral-free (A). Scale bars, 1 μm.
Figure 7.
Ultrastructure of proliferative cartilage of week 3 mouse tibial growth plates reveals matrix alterations in collagen X Tg mice. The territorial matrix of proliferative chondrocytes is shown. In controls (A), collagen fibrils are dispersed between a fine meshwork, likely composed of GAGs/PGs. In Tg mice (B), fibrils are difficult to distinguish because of abundant RHT-positive aggregates (dark dots). C and D: Higher magnifications of boxed-in zones in A and B, respectively. Note RHT-positive aggregates masking the collagen fibrils in D. Scale bars: 1 μm (A and B); 200 nm (C and D).
Figure 8.
Immunohistochemical localization of HA in growth plates of wild-type and collagen X Tg and KO mice. Longitudinal sections of week 3 tibiae from wild-type controls (WT, A and D), Tg mice **(**TG, B), KO mice (KO, E), and Tg (TG-Mut, C) or KO (KO-Mut, F) mice exhibiting perinatal lethality. Free HA was apparent as red rings encompassing hypertrophic chondrocytes in A, whereas background staining was minimal in D. In B, E, and F, only faint staining was seen around these cells. Moreover in C, minimal staining for HA was detected. Brackets approximate width of hypertrophic cartilage. Scale bar, 100 μm.
Figure 9.
Immunohistochemical localization of HSPG in growth plates of wild-type and collagen X Tg and KO mice. Longitudinal sections of week 3 tibiae from wild-type controls (WT, A and D), Tg mice **(**TG, B), KO mice (KO, E), and Tg (TG-Mut, C) or KO (KO-Mut, F) mice exhibiting perinatal lethality. Note HSPG localization to proliferative and hypertrophic cartilage, and to trabecular bone in A. In B, staining is pericellular in proliferative chondrocytes, and intensity is reduced in hypertrophic cartilage. In C, E, and F, staining is either faint or absent in the growth plate. D: Control where heparitinase was omitted. Brackets approximate width of hypertrophic cartilage. Scale bar, 100 μm.
Similar articles
- Chicken collagen X regulatory sequences restrict transgene expression to hypertrophic cartilage in mice.
Campbell MR, Gress CJ, Appleman EH, Jacenko O. Campbell MR, et al. Am J Pathol. 2004 Feb;164(2):487-99. doi: 10.1016/S0002-9440(10)63139-2. Am J Pathol. 2004. PMID: 14742255 Free PMC article. - Altered matrix at the chondro-osseous junction leads to defects in lymphopoiesis.
Sweeney E, Roberts D, Jacenko O. Sweeney E, et al. Ann N Y Acad Sci. 2011 Nov;1237:79-87. doi: 10.1111/j.1749-6632.2011.06227.x. Ann N Y Acad Sci. 2011. PMID: 22082369 Review. - Impact of mutations of cartilage matrix genes on matrix structure, gene activity and chondrogenesis.
So CL, Kaluarachchi K, Tam PP, Cheah KS. So CL, et al. Osteoarthritis Cartilage. 2001;9 Suppl A:S160-73. Osteoarthritis Cartilage. 2001. PMID: 11680681 - Chondrocyte cell death and intracellular distribution of COMP and type IX collagen in the pseudoachondroplasia growth plate.
Hecht JT, Makitie O, Hayes E, Haynes R, Susic M, Montufar-Solis D, Duke PJ, Cole WG. Hecht JT, et al. J Orthop Res. 2004 Jul;22(4):759-67. doi: 10.1016/j.orthres.2003.11.010. J Orthop Res. 2004. PMID: 15183431
Cited by
- Beta-catenin, cartilage, and osteoarthritis.
Wu Q, Zhu M, Rosier RN, Zuscik MJ, O'Keefe RJ, Chen D. Wu Q, et al. Ann N Y Acad Sci. 2010 Mar;1192(1):344-50. doi: 10.1111/j.1749-6632.2009.05212.x. Ann N Y Acad Sci. 2010. PMID: 20392258 Free PMC article. Review. - Matrix remodeling during endochondral ossification.
Ortega N, Behonick DJ, Werb Z. Ortega N, et al. Trends Cell Biol. 2004 Feb;14(2):86-93. doi: 10.1016/j.tcb.2003.12.003. Trends Cell Biol. 2004. PMID: 15102440 Free PMC article. Review. - Surviving endoplasmic reticulum stress is coupled to altered chondrocyte differentiation and function.
Tsang KY, Chan D, Cheslett D, Chan WC, So CL, Melhado IG, Chan TW, Kwan KM, Hunziker EB, Yamada Y, Bateman JF, Cheung KM, Cheah KS. Tsang KY, et al. PLoS Biol. 2007 Mar;5(3):e44. doi: 10.1371/journal.pbio.0050044. PLoS Biol. 2007. PMID: 17298185 Free PMC article. - Extracellular matrix degradation and remodeling in development and disease.
Lu P, Takai K, Weaver VM, Werb Z. Lu P, et al. Cold Spring Harb Perspect Biol. 2011 Dec 1;3(12):a005058. doi: 10.1101/cshperspect.a005058. Cold Spring Harb Perspect Biol. 2011. PMID: 21917992 Free PMC article. Review. - How cell migration helps immune sentinels.
Delgado MG, Lennon-Duménil AM. Delgado MG, et al. Front Cell Dev Biol. 2022 Oct 4;10:932472. doi: 10.3389/fcell.2022.932472. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36268510 Free PMC article. Review.
References
- Chan D, Jacenko O: Phenotypic and biochemical consequences of collagen X mutations in mice and humans. Matrix Biol 1998, 17:1169-1184 - PubMed
- Jacenko O, LuValle P, Olsen BR: Spondlylometaphyseal dysplasia in mice carrying a dominant negative mutation in a matrix protein specific for cartilage-to-bone transition. Nature 1993, 365:56-61 - PubMed
- Lunstrum GP, Keene DR, Weksler NB, Cho YJ, Cornwall M, Horton WA: Chondrocyte differentiation in a rat mesenchymal cell line. J Histochem Cytochem 1999, 47:1-6 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous