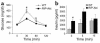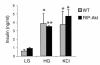Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia - PubMed (original) (raw)
Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia
E Bernal-Mizrachi et al. J Clin Invest. 2001 Dec.
Abstract
The phosphoinositide 3-kinase-Akt/PKB pathway mediates the mitogenic effects various nutrients and growth factors in cultured cells. To study its effects in vivo in pancreatic islet beta cells, we created transgenic mice that expressed a constitutively active Akt1/PKB alpha linked to an Insulin gene promoter. Transgenic mice exhibited a grossly visible increase in islet mass, largely due to proliferation of insulin-containing beta cells. Morphometric analysis verified a six-fold increase in beta cell mass/pancreas, a two-fold increase in 5-bromo-2'-deoxyuridine incorporation, a four-fold increase in the number of beta cells per pancreas area, and a two-fold increase in cell size in transgenic compared with wild-type mice at 5 weeks. At least part of the increase in beta cell number may be accounted for by neogenesis, defined by criteria that include beta cells proliferating from ductular epithelium, and by a six-fold increase in the number of single and doublet beta cells scattered throughout the exocrine pancreas of the transgenic mice. Glucose tolerance was improved, and fasting as well as fed insulin was greater compared with wild-type mice. Glucose-stimulated insulin secretion was maintained in transgenic mice, which were resistant to streptozotocin-induced diabetes. We conclude that activation of the Akt1/PKB alpha pathway affects islet beta cell mass by alteration of size and number.
Figures
Figure 1
Transgene expression in RIP-Akt mice. Expression of the transgene was demonstrated by immunofluorescent staining of the pancreas with an anti–HA-tag Ab (red). No signal was observed in pancreas from a control animal (data not shown).
Figure 2
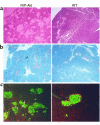
Macroscopic abnormalities from transgenic mice expressing a constitutively active Akt1/PKB. Macroscopic findings observed during pancreatic dissections of RIP-Akt (left) and wild-type (WT) (right) mice. Gross identification of islets in pancreas from a transgenic mouse (see arrow for example).
Figure 3
Morphological changes in pancreas from RIP-Akt mice. (a) Hematoxylin and eosin staining of pancreas from a transgenic (left) and control mouse (right), each 24 weeks of age. (b) Immunostaining of pancreas for insulin (red) in 5-week-old transgenic (left) and control mouse (right). Note multiple areas of single and doublet extra-islet β cells, indicated by arrow. (c) Altered islet architecture was demonstrated by immunofluorescence staining of pancreas with insulin (green) and non–β cells (red) in 5-week-old transgenic (left) and control mouse (right). Occasional cells expressing both insulin and a non–β cell hormone are indicated in yellow (see arrows for example).
Figure 4
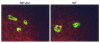
Assessment of proliferation and neogenesis in a pancreas from RIP-Akt mice. (a) β cell replication was demonstrated by BrdU incorporation (arrows) in cells stained for insulin (red) from transgenic (left) and control mouse (right). (b) Transgenic mice (left) showing evidence of neogenesis by β cells budding from ductal cells (arrow), as well as increased single and doublet β cells.
Figure 5
Alterations in pancreatic β cell size observed in RIP-Akt mice. Immunofluorescence for insulin (green) in pancreatic sections (same magnification) from transgenic (left) and control mouse (right). This section illustrates the increased β cell size in transgenic compared with WT mice.
Figure 6
Intraperitoneal glucose tolerance tests. Five-month-old mice were fasted 6 hours, then injected with glucose (2 mg/g body weight). Blood samples from the tail vein were obtained at the indicated times. Plasma glucose (a) (RIP-Akt, n = 4, and WT, n = 5) and insulin (b) (RIP-Akt, n = 3, and WT, n = 3). Results are presented as mean ± SEM. *P < 0.01.
Figure 7
Assessment of insulin secretion in isolated islets. Incubation of islets in the presence of 2 mM glucose (LG), 20 mM glucose (HG), and 30 mM KCl was performed as described in Methods. Islets were isolated from 2- to 3-month-old RIP-Akt (n = 4) and WT littermates (n = 3). Results are mean ± SEM. *P < 0.05; **P < 0.01.
Figure 8
Effect of STZ administration on blood glucose levels in RIP-Akt and control. Two- to three-month-old RIP-Akt (n = 5) and WT (n = 12) mice were subjected to intraperitoneal injections. STZ injections (arrows) were performed at the indicated doses. Results are expressed as mean ± SEM. *P < 0.01.
Comment in
- A kinase in the life of the beta cell.
Accili D. Accili D. J Clin Invest. 2001 Dec;108(11):1575-6. doi: 10.1172/JCI14454. J Clin Invest. 2001. PMID: 11733550 Free PMC article. No abstract available.
Similar articles
- Selective expansion of the beta-cell compartment in the pancreas of keratinocyte growth factor transgenic mice.
Wagner M, Koschnick S, Beilke S, Frey M, Adler G, Schmid RM. Wagner M, et al. Am J Physiol Gastrointest Liver Physiol. 2008 May;294(5):G1139-47. doi: 10.1152/ajpgi.00338.2007. Epub 2008 Mar 27. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18372394 - Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha.
Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. Tuttle RL, et al. Nat Med. 2001 Oct;7(10):1133-7. doi: 10.1038/nm1001-1133. Nat Med. 2001. PMID: 11590437 - Transgenic mice expressing Shb adaptor protein under the control of rat insulin promoter exhibit altered viability of pancreatic islet cells.
Welsh M, Christmansson L, Karlsson T, Sandler S, Welsh N. Welsh M, et al. Mol Med. 1999 Mar;5(3):169-80. Mol Med. 1999. PMID: 10404514 Free PMC article. - Overexpression of Pref-1 in pancreatic islet β-cells in mice causes hyperinsulinemia with increased islet mass and insulin secretion.
Wang Y, Lee K, Moon YS, Ahmadian M, Kim KH, Roder K, Kang C, Sul HS. Wang Y, et al. Biochem Biophys Res Commun. 2015 Jun 12;461(4):630-5. doi: 10.1016/j.bbrc.2015.04.078. Epub 2015 Apr 24. Biochem Biophys Res Commun. 2015. PMID: 25918019 Free PMC article.
Cited by
- Ginseng berry extract supplementation improves age-related decline of insulin signaling in mice.
Seo E, Kim S, Lee SJ, Oh BC, Jun HS. Seo E, et al. Nutrients. 2015 Apr 22;7(4):3038-53. doi: 10.3390/nu7043038. Nutrients. 2015. PMID: 25912041 Free PMC article. - Reduced expression of the LRP16 gene in mouse insulinoma (MIN6) cells exerts multiple effects on insulin content, proliferation and apoptosis.
Li X, Xue B, Wang X, Sun L, Zhang T, Qu L, Zou X, Mu Y. Li X, et al. J Huazhong Univ Sci Technolog Med Sci. 2012 Apr;32(2):190-198. doi: 10.1007/s11596-012-0034-6. Epub 2012 Apr 20. J Huazhong Univ Sci Technolog Med Sci. 2012. PMID: 22528219 - Integrated investigation and discovery of therapeutic targets for 3-hydroxybakuchiol against diabetes based on molecular docking studies and cell experiments.
Liu M, Wang X, Yang J, Qin D. Liu M, et al. BMC Complement Med Ther. 2023 Nov 29;23(1):431. doi: 10.1186/s12906-023-04248-6. BMC Complement Med Ther. 2023. PMID: 38031191 Free PMC article. - Insulin regulates the activity of forkhead transcription factor Hnf-3beta/Foxa-2 by Akt-mediated phosphorylation and nuclear/cytosolic localization.
Wolfrum C, Besser D, Luca E, Stoffel M. Wolfrum C, et al. Proc Natl Acad Sci U S A. 2003 Sep 30;100(20):11624-9. doi: 10.1073/pnas.1931483100. Epub 2003 Sep 19. Proc Natl Acad Sci U S A. 2003. PMID: 14500912 Free PMC article. - Akt isoforms and glucose homeostasis - the leptin connection.
Hay N. Hay N. Trends Endocrinol Metab. 2011 Feb;22(2):66-73. doi: 10.1016/j.tem.2010.09.003. Epub 2010 Oct 12. Trends Endocrinol Metab. 2011. PMID: 20947368 Free PMC article. Review.
References
- Steil GM, et al. Adaptation of beta-cell mass to substrate oversupply: enhanced function with normal gene expression. Am J Physiol Endocrinol Metab. 2001;280:E788–E796. - PubMed
- Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44:249–256. - PubMed
- Garcia-Ocana A, et al. Hot topic—using beta-cell growth factors to enhance human pancreatic islet transplantation. J Clin Endocrinol Metab. 2001;86:984–988. - PubMed
- Swenne I. The role of glucose in the in vitro regulation of cell cycle kinetics and proliferation of fetal pancreatic B-cells. Diabetes. 1982;31:754–760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous