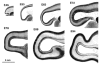Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey - PubMed (original) (raw)
Comparative Study
Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey
Iain H M Smart et al. Cereb Cortex. 2002 Jan.
Abstract
We examined the development of the occipital lobe in fetal monkeys between embryonic day 37 (E37) and E108 in Nissl-stained and acetylcholine esterase (AChE)-reacted sections. We paid particular attention to features that distinguish the development of presumptive area 17. At E46 the neuroepithelium consists of a ventricular zone and a monolayer cortical plate sandwiched between a thin marginal zone and a minimal presubplate. Between E55 and E65 an augmented subplate emerges and continues to expand up to E94 to become a major compartment of the developing cortex. A mitotic subventricular zone is established by E55. Peaking in depth at E72, it constitutes the principal germinal zone. By E78 an invading fibre tract divides it into an outer radially organized zone and a more conventional inner zone. AChE staining reveals the future area 17/18 border from E86 onwards. Proceeding from presumptive area 17 to area 18 there is a progressive thinning of the radially structured subventricular zone. Comparison of these results with corticogenesis in rodents suggests a number of potentially unique primate features: (i) a minimal preplate stage; (ii) a radially augmented germinal zone not previously described in non-primates; (iii) a fibre tract dividing the subventricular zone into two laminae; (iv) late generation and expansion of the subplate.
Figures
Figure 1
Parasagittal sections of the occipital lobe at different stages of development between E46 and E94. Note the increase in depth of the germinal zone at the occipital pole of the hemisphere between E65 and E72. Note also the subdivision of the germinal zone between E72 and E78 into an inner and outer part by an intruding fibre tract.
Figure 2
Transects of presumptive area 17 taken from the brains shown in Figure 1. An early appearing outer fibre layer (OFL) with a distinctly pallisaded appearance forms a major landmark throughout the period of development. The ventricular zone (VZ) declines progressively after E65. The subventricular zone, by contrast, increases progressively in depth and by E72 is divided into an inner subventricular zone (ISVZ) and outer subventricular zone (OSVZ) by an intruding inner fibre layer (IFL). The increase in the OSVZ is particularly important between E65 and E72 and occurs as the VZ declines. The cortical plate (CP), or its ‘pioneer plate precursor’ (Meyer et al., 2000), appears as early as E46. It increases progressively in depth and shows little change in packing density of its cells until after E88. Little in the way of the ‘presubplate’ of Kostovic and Rakic is evident in this type of preparation (Kostovic and Rakic, 1990). The subplate proper (SP), however, is evident after E55. Subsequently it increases in depth in two stages: between E65 and E72 the increase is densely cellular in character, and between E72 and E78 the increase appears to be derived mainly from a decrease in the packing density of the component cells. The marginal zone (MZ) is minimal before E65. A curiously conspicuous ‘clear layer’, marked by an asterisk, located in the deep SP, is transiently present at E72. It may be a new feature or an elaboration of the original ‘presubplate’. At later stages it appears to merge into the SP.
Figure 3
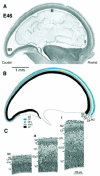
Rostrocaudal gradient of cortical development at E46. (A) Photomicrograph of a parasagittal section of an E46 Nissl-stained section. (B) Drawing of above identifying various layers. (C) High-power views of transects taken through levels I (rostral), II (dorsal) and III (caudal) in (A). Note the rostrocaudal gradient of decreasing depth of all layers except the VZ. At the caudal pole they are of minimal depth and, compared to more rostral areas, ill-defined. Abbreviations as in Figure 2.
Figure 4
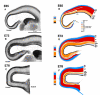
High-power photomicrographs of parasagittal sections. On the left are photomicrographs through the caudal pole of cerebral hemispheres at stated ages. On the right are matching drawings outlining various layers identified by reference to colour coding set out in the inset diagram. Abbreviations as in Figure 2.
Figure 5
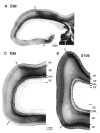
Photomicrographs at higher-power of the germinal zones at E78. (A) Note the radial texture of the dominant OSVZ and of the lesser VZ. Note also the IFL separating the radial OSVZ from the more irregularly orientated nuclei of the ISVZ. (B) Higher-power view of the OSVZ. Note radial texture of tissue and the presence of mitotic figures. (C) View of the ISVZ and VZ. Note the presence of mitotic figures among the irregularly orientated nuclei of the ISVZ. In the VZ, nuclei are radially arranged and mitotic figures lie towards the ventricular surface, indicating the continuation of interkinetic nuclear migration.
Figure 6
Acetylcholinesterase reactivity in the occipital lobe and thalamus. Dark bars show the 17/18 border at E86 and E106. At all ages there is a strong labelling of fibres in OFL. Note that AchE reactivity reveals lamination of the lateral geniculate nucleus at this early developmental stage. Abbreviations as in Figure 2.
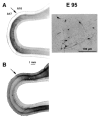
Figure 7
Identification of subplate and areal borders at E95. (A) Neuropeptide Y labelling identifies the subplate. Low magnification (left panel) shows that there is a difference in the intensity of labelling in the region of the putative area 17/18 border. This is due to changes in the density of labelled subplate neurons (right panel). (B) Adjacent section reacted for acetylcholinesterase activity. This confirms the location of the area 17/18 border (for further details, see Fig. 6).
Figure 8
Comparison of histological sequences in mouse and monkey telencephalic wall. These drawings are of transects through putative Area 17 in (A) monkey and (B) mouse at comparable developmental stages. The depth of each layer is drawn to a common scale. The internal detail of each layer is not to scale but depicts the orientation, shape and relative packing density of nuclei in each layer. The drawings of monkey neuroepithelium were taken from the present material. The drawings from the mouse cortex were of material from the library of serially sectioned, Bouin-fixed mouse embryonic brains prepared for previous studies by I.H.M.S. The vertically aligned pairs have been chosen with reference to birthdating experiments so as to illustrate corticogenesis at equivalent developmental stages (Angevine and Sidman, 1961; Rakic, 1974; Smart and Smart, 1982; Caviness et al., 1995). Abbreviations and color coding as in Figures 2–4.
Similar articles
- Unique profiles of the alpha 1-, alpha 2-, and beta-adrenergic receptors in the developing cortical plate and transient embryonic zones of the rhesus monkey.
Lidow MS, Rakic P. Lidow MS, et al. J Neurosci. 1994 Jul;14(7):4064-78. doi: 10.1523/JNEUROSCI.14-07-04064.1994. J Neurosci. 1994. PMID: 8027763 Free PMC article. - Early expression of GABA-containing neurons in the prefrontal and visual cortices of rhesus monkeys.
Schwartz ML, Meinecke DL. Schwartz ML, et al. Cereb Cortex. 1992 Jan-Feb;2(1):16-37. doi: 10.1093/cercor/2.1.16. Cereb Cortex. 1992. PMID: 1633406 - Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain.
Kostovic I, Rakic P. Kostovic I, et al. J Comp Neurol. 1990 Jul 15;297(3):441-70. doi: 10.1002/cne.902970309. J Comp Neurol. 1990. PMID: 2398142 - Populations of subplate and interstitial neurons in fetal and adult human telencephalon.
Judaš M, Sedmak G, Pletikos M, Jovanov-Milošević N. Judaš M, et al. J Anat. 2010 Oct;217(4):381-99. doi: 10.1111/j.1469-7580.2010.01284.x. J Anat. 2010. PMID: 20979586 Free PMC article. Review. - Calcium-binding proteins in the human developing brain.
Ulfig N. Ulfig N. Adv Anat Embryol Cell Biol. 2002;165:III-IX, 1-92. Adv Anat Embryol Cell Biol. 2002. PMID: 12236093 Review.
Cited by
- Epigenomic landscapes during prefrontal cortex development and aging in rhesus.
Ning C, Wu X, Zhao X, Lu Z, Yao X, Zhou T, Yi L, Sun Y, Wu S, Liu Z, Huang X, Gao L, Liu J. Ning C, et al. Natl Sci Rev. 2024 Jun 18;11(8):nwae213. doi: 10.1093/nsr/nwae213. eCollection 2024 Aug. Natl Sci Rev. 2024. PMID: 39183748 Free PMC article. - The Principle of Cortical Development and Evolution.
Yang Z. Yang Z. Neurosci Bull. 2024 Jul 18. doi: 10.1007/s12264-024-01259-2. Online ahead of print. Neurosci Bull. 2024. PMID: 39023844 Review. - A molecular and cellular perspective on human brain evolution and tempo.
Lindhout FW, Krienen FM, Pollard KS, Lancaster MA. Lindhout FW, et al. Nature. 2024 Jun;630(8017):596-608. doi: 10.1038/s41586-024-07521-x. Epub 2024 Jun 19. Nature. 2024. PMID: 38898293 Review. - Gene regulatory landscape of cerebral cortex folding.
Singh A, Del-Valle-Anton L, de Juan Romero C, Zhang Z, Ortuño EF, Mahesh A, Espinós A, Soler R, Cárdenas A, Fernández V, Lusby R, Tiwari VK, Borrell V. Singh A, et al. Sci Adv. 2024 Jun 7;10(23):eadn1640. doi: 10.1126/sciadv.adn1640. Epub 2024 Jun 5. Sci Adv. 2024. PMID: 38838158 Free PMC article. - The meso-connectomes of mouse, marmoset, and macaque: network organization and the emergence of higher cognition.
Magrou L, Joyce MKP, Froudist-Walsh S, Datta D, Wang XJ, Martinez-Trujillo J, Arnsten AFT. Magrou L, et al. Cereb Cortex. 2024 May 2;34(5):bhae174. doi: 10.1093/cercor/bhae174. Cereb Cortex. 2024. PMID: 38771244 Free PMC article. Review.
References
- Anderson SA, Eisenstat DD, Shi L, Rubenstein LR. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources