M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain - PubMed (original) (raw)
M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain
E C Cooper et al. J Neurosci. 2001.
Abstract
Mutations in the potassium channel subunit KCNQ2 lead to benign familial neonatal convulsions, a dominantly inherited form of generalized epilepsy. In heterologous cells, KCNQ2 expression yields voltage-gated potassium channels that activate slowly (tau, approximately 0.1 sec) at subthreshold membrane potentials. KCNQ2 associates with KCNQ3, a homolog, to form heteromeric channels responsible for the M current (I(M)) in superior cervical ganglion (SCG) neurons. Muscarinic acetylcholine and peptidergic receptors inhibit SCG I(M), causing slow EPSPs and enhancing excitability. Here, we use KCNQ2N antibodies, directed against a conserved N-terminal portion of the KCNQ2 polypeptide, to localize KCNQ2-containing channels throughout mouse brain. We show that KCNQ2N immunoreactivity, although widespread, is particularly concentrated at key sites for control of rhythmic neuronal activity and synchronization. In the basal ganglia, we find KCNQ2N immunoreactivity on somata of dopaminergic and parvalbumin (PV)-positive (presumed GABAergic) cells of the substantia nigra, cholinergic large aspiny neurons of the striatum, and GABAergic and cholinergic neurons of the globus pallidus. In the septum, GABAergic, purinergic, and cholinergic neurons that contribute to the septohippocampal and septohabenular pathways exhibit somatic KCNQ2 labeling. In the thalamus, GABAergic nucleus reticularis neurons that regulate thalamocortical oscillations show strong labeling. In the hippocampus, many PV-positive and additional PV-negative interneurons exhibit strong somatic staining, but labeling of pyramidal and dentate granule somata is weak. There is strong neuropil staining in many regions. In some instances, notably the hippocampal mossy fibers, evidence indicates this neuropil staining is presynaptic.
Figures
Fig. 1.
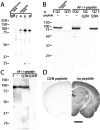
Characterization of anti-KCNQ2N antisera and affinity-purified antibodies. A, Western blots using preimmune and immune sera against KCNQ2 expressed in HEK cells. Immunoreactivity against the expressed ∼85 kDa KCNQ2 band is absent in preimmune sera and increases progressively during the immunization.B, Immune sera detects the HEK-expressed KCNQ2 protein but not KCNQ3 (∼90 kDa; data not shown). After affinity purification (AP), an ∼70 kDa band background band is eliminated, and recognition of transfected KCNQ2 is abolished by the KCNQ2N peptide but not by a peptide from the KCNQ3 N terminus. C, KCNQ2N antibodies detect two bands of ∼85 and ∼90 kDa in mouse brain membranes. D, Immunoperoxidase staining by KCNQ2N antibodies is abolished by preincubation of the antibodies with the immunogenic peptide. Scale bar, 1 mm.
Fig. 2.
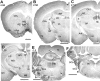
Distribution of KCNQ2N immunoreactivity in the mouse brain. A, Regions shown are the piriform cortex (Pir), caudoputamen (CPu), ventral pallidum (VPal), island of Calleja major (IcjM), and medial septum (MS) and diagonal band of Broca (dbB). B, Section through the globus pallidus (GP), reticular (Rt), laterodorsal (LD), and ventrolateral (VL) thalamus, hippocampal mossy fibers (MF), and lateral hypothalamus (LH). C, Heavy staining of the mossy fibers and habenula (Hb); staining in the fimbria (fi), ventroposterior thalamus (VP), amygdala (Am), and lateral hypothalamus (LH) is lighter or moderate.D, Staining in the subiculum (Sb), mammillary body (MB), zona incerta (Zi), and anterior pretectal (APt), lateral geniculate (LG), and rostral interstitial (RI) nuclei. The amygdala and medial geniculate (MG) regions show light staining. E, The anteroventral cochlear nucleus (AVCo), sensory division of the trigeminal nucleus (5s), superior olive (SO), trapezoid body (tz), dorsal tegmentum (Dtg), Purkinje cell layer (PC), and inferior colliculus (IC) show moderate to heavy cell body staining. White matter tracts, including the middle cerebellar peduncle (mcp) and facial (7n) and vestibulocochlear (8n) nerves are unlabeled. F, The vestibular (Ve), dorsal cochlear (DCo), and dentate, interposed, and medial cerebellar (DCb, Icb,MCb) nuclei all show heavy staining, but the facial nucleus (7) is unstained. _Numbers_below the panel letters indicate rostrocaudal positions of the sections according to the atlas of Franklin and Paxinos (1997). Scale bars, 1 mm.
Fig. 3.
Distribution of KCNQ2N immunoreactivity in the olfactory bulb, ventral forebrain, and hippocampus. A, In olfactory bulb, strong neuropil staining is detected in the glomerular layer. Periglomerular cells in the glomerular layer (Gl) and mitral cells (Mit) exhibit strong somatic staining. Staining is lighter in the external plexiform (ePl), internal plexiform (iPl),and granule cell (GC) layers. B, In the basal forebrain, strong neuropil and somatic staining is apparent in the medial septum (MS), diagonal band of Broca (dbB), ventral pallidum (VPal), and island of Calleja major (IcjM). The anterior commissure (AC) and caudoputamen (CPu) are indicated. C, In the hippocampal formation, neuropil staining is intense along the mossy fiber pathway in the hilus (h) of the dentate gyrus (DG) and stratum lucidum (inset, sl) of CA3. Moderate neuropil staining is present in the stratum oriens (so), stratum radiatum (sr), and stratum lacunosum-moleculare (sl) of the hippocampus and the dentate molecular layer (m). In the stratum pyramidale (sp) and stratum granulosum (g), staining is light, except for a few cell bodies (e.g., CA3 inset). Similar profiles are seen in the stratum pyramidale of CA1 and in the dentate granule cell layer (Fig. 4). Scale bars: A–C, 250 μm;inset, 125 μm.
Fig. 4.
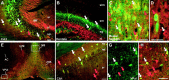
KCNQ2N immunolocalization in the hippocampus and basal forebrain. A–C, E–G, Immunofluorescence of sections double-labeled with KCNQ2N (red) and PV (green).A, In CA3, KCNQ2N intensely labels mossy fiber (MF) bundles in the stratum lucidum (sl) and, less strongly, neuropil of the stratum radiatum (sr) and stratum oriens (so). Interneurons in the stratum pyramidale (sp) and oriens are double-labeled by PV and KCNQ2N (white arrows). Additional interneurons in the stratum oriens and radiatum (e.g.,red arrow) are KCNQ2N-positive but PV-negative.B, In the dentate gyrus, KCNQ2N stains hilar mossy fiber and PV-positive interneurons in the granule cell layer (sg). The stratum lacunosum-moleculare (slm), stratum moleculare (sm), and hilus (hi) are indicated. C, Detail of_E_. The medial septum contains numerous PV-positive (white arrows) and PV-negative cells labeled with KCNQ2N. D, Colabeling in the medial septum by ChAT (green) and KCNQ2N (red); the_red arrow_ indicates a colabeled cell; the white arrow indicates a KCNQ2N-immunoreactive, ChAT-nonreactive profile. E, Low-power view of septal region (as in Fig.3_B_). The anterior commissure (AC) lacks KCNQ2N immunostaining, but the medial septum (MS), island of Calleja major (IcjM), dbB, and ventral pallidum (VPal) show strong somatic and neuropil staining. Boxes indicate regions shown at higher magnification in C, G, and_H_. F, In CA1, PV-positive (white arrows) and PV-negative (red arrows) interneurons in the stratum oriens, pyramidale, and radiatum show moderate labeling for KCNQ2N. G, H, PV and KCNQ2N double staining of the ventral pallidum. In G, only PV staining is shown; in H, KCNQ2N (red) and PV (green) staining are superimposed. Red arrows indicate position of two of the numerous PV-negative, KCNQ2N-positive cell bodies; white arrows show colabeled cells. Scale bars: E, 250 μm; F(applies to A, B), C, D, G, H, 50 μm.
Fig. 5.
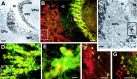
KCNQ2N immunostaining of the triangular septal nucleus (TS) and habenula. A, KCNQ2N strongly stained somata of tightly packed granular neurons in the triangular nucleus (inset shows higher magnification) and lightly stained the neuropil in the lateral dorsal septal nucleus (LSD). The fibers of the hippocampal commissure (HC) are unstained. LV, Lateral ventricle;Cg, cingulate gyms. B, Immunoperoxidase staining of habenula, showing intensely reactive somata in the medial (MH) and lateral (LH) regions. C, Colabeling for KCNQ2N (red) and ChAT (green) is exhibited by the cholinergic neurons of the medial habenula but not the neurons of the lateral habenula. The fasciculus retroflexus (FR) the projection pathway of the medial habenula to the interpeduncular nucleus, is strongly ChAT-immunoreactive. D, Colabeling with KCNQ2 (red) and PV (green) showing that cholinergic KCNQ2-positive medial habenula neurons are PV-negative, as expected. PV staining of the lateral habenula neuropil is intense, and some lateral habenula somata appear to contain both KCNQ2N and PV immunoreactivity. Scale bars: A, 250 μm;inset, B–D, 50 μm.
Fig. 6.
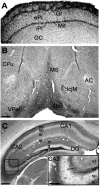
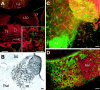
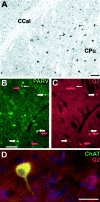
KCNQ2N immunolocalization in the caudoputamen (CPu). A, Immunoperoxidase stain showing moderate neuropil staining and moderate to heavy staining of somata and proximal dendrites in the CPu, but little staining in the corpus callosum (CCal). B, C, Double immunolabeling for PV (PARV, green) and KCNQ2N (red) in the lateral caudoputamen. Many cells are labeled strongly by PV and weakly by KCNQ2N (e.g., those indicated by_wide white arrows_). A few, typically larger, cells are labeled by KCNQ2N but not by PV (red arrows) or PV but not KCNQ2N (thin white arrow). D, Double immunolabeling for choline acetyltransferase (green) and KCNQ2N (red) in the caudo putamen. Many cells exhibit staining by both antibodies, but some are stained weakly for KCNQ2 only. Cell nuclei are counterstained with DAPI (blue). Scale bars, 100 μm.
Fig. 7.
KCNQ2N immunolocalization in the globus pallidus (GP) and nucleus reticularis thalami (Rt).A, Immunoperoxidase stain using KCNQ2N antibodies shows strong staining of neuronal profiles in the CPu, GP externa and interna (Gpe, Gpi), and Rt, but not in neighboring thalamic relay nuclei, ventroposterior lateral (VPl) and ventroposterior medial (VPm), or the internal capsule (IC). B, Double immunolabeling of a similar section to A, using KCNQ2N and PV antibodies. Double-labeled neurons (orange to yellow) are numerous in the nRT and Gpe. In addition, a few neurons (red arrows) in the IC show labeling for KCNQ2N but not PV.C, Immunoperoxidase staining with VAchT antibodies reveals the distribution of cholinergic neurons in and near the Gpe. VAchT labels neuropil strongly in the CPu and moderately in the Gpe and Rt. Intensely labeled neurons are preferentially located at periphery of and ventral to the Gpe. D, Higher magnification view of Rt, showing that all PV-labeled neurons are also KCNQ2N labeled.E, Detail of D, showing punctate appearance of KCNQ2N staining. F, Higher magnification view of region near border of IC and ventral border of Gpe (location indicated by_box_ in C), after double immunolabeling with KCNQ2N (red) and ChAT (green). Neuropil and neurons of Gpe are labeled by KCNQ2N only, but a few neurons (arrows) at border are stained by both antibodies.G, Higher magnification views of Gpe double-labeled with PV (green) and KCNQ2N (red). Most cells appear_yellow_ or orange because of colabeling with both markers, but a few preferentially located near periphery of Gpe are labeled by KCNQ2 only (arrows). Scale bars: A, 250 μm; B, C, 100 μm; D, 25 μm;E, 10 μm; F, G, 50 μm.
Fig. 8.
Regions of high KCNQ2N immunoreactivity in thalamus and brainstem. A, Ventral midbrain. Staining is detected in the substantia nigra reticulata (SNr) and red (Rd), interpeduncular (IP), and oculomotor (3) nuclei. The medial lemniscus (ml) and oculomotor nerve (3n) are unstained. B, Heavy staining is shown in the zona incerta and substantia nigra reticulata, but the cerebral peduncles and medial longitudinal fasciculus (Cped, MLF) are not stained.C, The anteroventral cochlear nucleus (AVCo) shows somatic staining, but the nearby cochlear nerve and spinal tract of the trigeminal nucleus (8n, 5n) are unstained. D, Substantia nigra compacta (SNc), substantia nigra reticulata, and ventral tegmental nuclei (VTA) showing somatic staining. Scale bars: A, 500 μm; B–D, 250 μm.
Fig. 9.
Distribution of KCNQ2N immunoreactivity in the cerebellum. A, Immunoperoxidase stain showing strong staining of Purkinje cell (pc) somata and molecular level neuropil (m), with light staining of the granule cell layer (gc) and absent staining of deep cerebellar white matter (wm). Scattered cells in the granule cell layer exhibit moderate somatic staining.B, Higher-magnification view showing that immunopositive granule cell layer neurons are stained on their somata and apical dendrites. Staining of dendrites of Purkinje cells is not apparent.C, Double immunofluorescence image (red, KCNQ2N; green, PV), showing that both markers label Purkinje cells, but KCNQ2N-positive granule cell layer neurons are PV-negative, as expected for Golgi cells. Purkinje cell somata are surrounded by PV-positive axons and termini derived from inhibitory basket and stellate cells whose somata are located in the molecular layer. Scale bars: A, 250 μm; B, 100 μm; C, 50 μm.
Fig. 10.
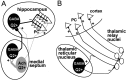
Distribution of KCNQ2N immunoreactivity in septohippocampal and thalamocortical neuronal networks.A, In the medial septum, KCNQ2N-immunoreactive (Q2+) GABAergic projection neurons receive excitatory inputs from nearby Q2+ cholinergic neurons. Both sets of septal neurons project to the hippocampus. Septal cholinergic fibers innervate all cell types in the hippocampus, but septal GABAergic fibers selectively innervate hippocampal interneurons (some Q2+) that arborize broadly to control activity and synchronization of hippocampal principal cells.B, In the thalamocortical network, glutamatergic thalamic relay neurons receive excitatory sensory inputs, and project to pyramidal cells (PC) in the sensory cortex, with collateral output to the _Q2_+ GABAergic reticular thalamic neurons. Thalamic reticular neurons project back to the relay nuclei, thereby helping synchronize prominent rhythmic firing associated with drowsiness and sleep. Ach, Acetylcholine.
Similar articles
- Colocalization and coassembly of two human brain M-type potassium channel subunits that are mutated in epilepsy.
Cooper EC, Aldape KD, Abosch A, Barbaro NM, Berger MS, Peacock WS, Jan YN, Jan LY. Cooper EC, et al. Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4914-9. doi: 10.1073/pnas.090092797. Proc Natl Acad Sci U S A. 2000. PMID: 10781098 Free PMC article. - Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium.
Hadley JK, Passmore GM, Tatulian L, Al-Qatari M, Ye F, Wickenden AD, Brown DA. Hadley JK, et al. J Neurosci. 2003 Jun 15;23(12):5012-9. doi: 10.1523/JNEUROSCI.23-12-05012.2003. J Neurosci. 2003. PMID: 12832524 Free PMC article. - Antibodies and a cysteine-modifying reagent show correspondence of M current in neurons to KCNQ2 and KCNQ3 K+ channels.
Roche JP, Westenbroek R, Sorom AJ, Hille B, Mackie K, Shapiro MS. Roche JP, et al. Br J Pharmacol. 2002 Dec;137(8):1173-86. doi: 10.1038/sj.bjp.0704989. Br J Pharmacol. 2002. PMID: 12466226 Free PMC article. - KCNQ2/KCNQ3 K+ channels and the molecular pathogenesis of epilepsy: implications for therapy.
Rogawski MA. Rogawski MA. Trends Neurosci. 2000 Sep;23(9):393-8. doi: 10.1016/s0166-2236(00)01629-5. Trends Neurosci. 2000. PMID: 10941184 Review. - [Genetic background of epilepsies].
Kelemen A, Szucs A, Rásonyi G, Janszky J, Holló A, Halász P. Kelemen A, et al. Ideggyogy Sz. 2004 May 20;57(5-6):141-51. Ideggyogy Sz. 2004. PMID: 15264690 Review. Hungarian.
Cited by
- KCNQ channels determine serotonergic modulation of ventral surface chemoreceptors and respiratory drive.
Hawryluk JM, Moreira TS, Takakura AC, Wenker IC, Tzingounis AV, Mulkey DK. Hawryluk JM, et al. J Neurosci. 2012 Nov 21;32(47):16943-52. doi: 10.1523/JNEUROSCI.3043-12.2012. J Neurosci. 2012. PMID: 23175845 Free PMC article. - New perspectives in brain information processing.
Nobili R. Nobili R. J Biol Phys. 2009 Oct;35(4):347-60. doi: 10.1007/s10867-009-9163-y. Epub 2009 Jun 4. J Biol Phys. 2009. PMID: 19669416 Free PMC article. - Ethanol inhibition of m-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons.
Koyama S, Brodie MS, Appel SB. Koyama S, et al. J Neurophysiol. 2007 Mar;97(3):1977-85. doi: 10.1152/jn.00270.2006. Epub 2006 Sep 6. J Neurophysiol. 2007. PMID: 16956995 Free PMC article. - The subthreshold-active KV7 current regulates neurotransmission by limiting spike-induced Ca2+ influx in hippocampal mossy fiber synaptic terminals.
Martinello K, Giacalone E, Migliore M, Brown DA, Shah MM. Martinello K, et al. Commun Biol. 2019 Apr 26;2:145. doi: 10.1038/s42003-019-0408-4. eCollection 2019. Commun Biol. 2019. PMID: 31044170 Free PMC article. - Expression and localization of K channels KCNQ2 and KCNQ3 in the mammalian cochlea.
Jin Z, Liang GH, Cooper EC, Jarlebark L. Jin Z, et al. Audiol Neurootol. 2009;14(2):98-105. doi: 10.1159/000158538. Epub 2008 Oct 1. Audiol Neurootol. 2009. PMID: 18827480 Free PMC article.
References
- Adams PR, Jones SW, Pennefather P, Brown DA, Koch C, Lancaster B. Slow synaptic transmission in frog sympathetic ganglia. J Exp Biol. 1986;124:259–285. - PubMed
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. Academic; San Diego: 1995. pp. 433–495.
- Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol. 1997;378:454–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases