Glycosylation alters steady-state inactivation of sodium channel Nav1.9/NaN in dorsal root ganglion neurons and is developmentally regulated - PubMed (original) (raw)
Glycosylation alters steady-state inactivation of sodium channel Nav1.9/NaN in dorsal root ganglion neurons and is developmentally regulated
L Tyrrell et al. J Neurosci. 2001.
Abstract
Na channel NaN (Na(v)1.9) produces a persistent TTX-resistant (TTX-R) current in small-diameter neurons of dorsal root ganglia (DRG) and trigeminal ganglia. Na(v)1.9-specific antibodies react in immunoblot assays with a 210 kDa protein from the membrane fractions of adult DRG and trigeminal ganglia. The size of the immunoreactive protein is in close agreement with the predicted Na(v)1.9 theoretical molecular weight of 201 kDa, suggesting limited glycosylation of this channel in adult tissues. Neonatal rat DRG membrane fractions, however, contain an additional higher molecular weight immunoreactive protein. Reverse transcription-PCR analysis did not show additional longer transcripts that could encode the larger protein. Enzymatic deglycosylation of the membrane preparations converted both immunoreactive proteins into a single faster migrating band, consistent with two states of glycosylation of Na(v)1.9. The developmental change in the glycosylation state of Na(v)1.9 is paralleled by a developmental change in the gating of the persistent TTX-R Na(+) current attributable to Na(v)1.9 in native DRG neurons. Whole-cell patch-clamp analysis demonstrates that the midpoint of steady-state inactivation is shifted 7 mV in a hyperpolarized direction in neonatal (postnatal days 0-3) compared with adult DRG neurons, although there is no significant difference in activation. Pretreatment of neonatal DRG neurons with neuraminidase causes an 8 mV depolarizing shift in the midpoint of steady-state inactivation of Na(v)1.9, making it indistinguishable from that of adult DRG neurons. Our data show that extensive glycosylation of rat Na(v)1.9 is developmentally regulated and changes a critical property of this channel in native neurons.
Figures
Fig. 1.
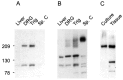
Immunoblot assay to show specificity of anti-Nav1.9 antibody. A, An immunoreactive band of molecular weight ∼210 kDa is detected in DRG and trigeminal (Trig.) ganglia but not in spinal cord (Sp. C) or liver. A much smaller nonspecific protein of molecular weight ∼100 kDa is detected in some samples. B, The blot was stripped and reprobed with a generic sodium channel antibody. DRG, trigeminal, and spinal cord samples show multiple immunoreactive bands, consistent with the presence of multiple sodium channels in these tissues. A number of smaller proteins are also detected and are the result of nonspecific interaction with the secondary antibody. C, Membrane fractions from adult DRG (Tissue) and from 24 hr cultured DRG cells (Culture). A single immunoreactive band of ∼210 kDa is detected in the cultured DRG sample, consistent with the conclusion that the ∼100 kDa protein is unrelated to Nav1.9. Positions of molecular weight markers 209, 130, and 78 kDa are indicated.
Fig. 2.
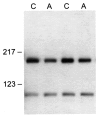
Axotomy reduces the level of Nav1.9 protein in rat DRG. The sciatic nerves of the right side of four rats were transected, and the level of Nav1.9 protein in the axotomized DRG (A) was compared with that in the intact ganglia (C) 14 d after axotomy in an immunoblot assay. The L4 and L5 DRG from the intact (C) and from the axotomized (A) sides were pooled from two rats, and the membrane fraction was analyzed using the Nav1.9-specific antibody. The intensity of the bands was determined by densitometry. Axotomy results in a clear reduction of the level of Nav1.9 protein in axotomized DRG. The nonspecific ∼100 kDa protein serves as a convenient marker to demonstrate equal loading of the gel. Positions of molecular weight markers 217 and 123 kDa are indicated.
Fig. 3.
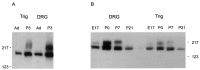
Two Nav1.9 immunoreactive bands are present in neonatal rat DRG. A, Membrane fractions from DRG and trigeminal ganglia (Trig) of P3 and adult (Ad) rats were probed with the Nav1.9 antibody and show an additional, higher molecular weight immunoreactive protein in the P3 sample compared with the adult sample. B, DRG and trigeminal ganglia membrane fractions from P0 and P7 rats contain two immunoreactive proteins. Trace amount of the slower migrating band is present in the E17 sample, whereas the P21 DRG sample contains only the faster migrating species.
Fig. 4.
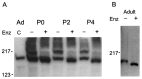
Both Nav1.9 immunoreactive proteins are glycosylated. A, Treatment of the membrane fractions from P0, P2, and P4 DRG with the glycosidase PNGase F (Enz) converts the immunoreactive proteins into a single faster migrating species. Samples were separated on a 4–15% gradient gel before electroblotting. Ad shows the immunoreactive protein from untreated adult DRG sample. B, Similar treatment of the membrane fraction from adult DRG converts the ∼210 kDa protein into a faster migrating species. Samples were separated on a 5% gel before electroblotting. The + and − signs indicate treated and untreated samples, respectively.
Fig. 5.
Separation of Nav1.9 Na+ currents from Nav1.8 Na+ currents by prepulse subtraction in adult and neonatal DRG neurons. A, Families of Na+ current traces from an adult small-diameter DRG neuron in the presence of 300 n
m
TTX. The capacitance of the adult DRG neuron was 13.31 pF. Both Nav1.9 Na+ current and Nav1.8 Na+ current were elicited in response to 100 msec test pulses from a holding potential of −130 mV to test potentials ranging from −100 to + 60 mV in 5 mV steps. B, The same neuron when given a prepulse of −50 mV for 500 msec before the test potentials from −100 to +60 mV elicited only Nav1.8 Na+ currents. C, Subtraction of current traces shown in B from the current traces shown in A yields Nav1.9 Na+currents. D–F represent separation of Nav1.9 Na+ currents from Nav1.8 Na+ currents in a neonatal DRG neuron as shown in A–C. The capacitance of the neonatal DRG neuron was 7.13 pF.
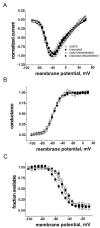
Fig. 6.
Desialidation affects the steady-state inactivation of Nav1.9 Na+ currents only in neonatal DRG neurons. A, Normalized current–voltage curves for neonatal (filled circles), adult (open circles), neonatal desialidated (filled squares), and adult desialidated (open squares) Nav1.9 Na+currents are shown in A. Current values were normalized to the peak value obtained at −40 mV. Symbols are the same for B and C and are shown also in the figure for clarity. B, Desialidation did not affect voltage dependence of activation of neonatal Nav1.9 Na+ currents. Voltage dependence of activation of neonatal, adult, neonatal desialidated, and adult desialidated Nav1.9 channels are shown. C, Desialidation causes a depolarizing shift of steady-state inactivation of neonatal Nav1.9 Na+ currents. Steady-state voltage dependence of inactivation of neonatal, adult, neo- natal desialidated, and adult desialidated Nav1.9 channels are shown. Lines are the Boltzmann fits to the data. The values of_V_1/2 and slope from Boltzmann fits of steady-state activation and steady-state inactivation of pooled neonatal, adult, neonatal desialidated, and adult desialidated Nav1.9 Na+ currents are shown in Table1.
Similar articles
- Changes in expression of two tetrodotoxin-resistant sodium channels and their currents in dorsal root ganglion neurons after sciatic nerve injury but not rhizotomy.
Sleeper AA, Cummins TR, Dib-Hajj SD, Hormuzdiar W, Tyrrell L, Waxman SG, Black JA. Sleeper AA, et al. J Neurosci. 2000 Oct 1;20(19):7279-89. doi: 10.1523/JNEUROSCI.20-19-07279.2000. J Neurosci. 2000. PMID: 11007885 Free PMC article. - Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons.
Cummins TR, Black JA, Dib-Hajj SD, Waxman SG. Cummins TR, et al. J Neurosci. 2000 Dec 1;20(23):8754-61. doi: 10.1523/JNEUROSCI.20-23-08754.2000. J Neurosci. 2000. PMID: 11102483 Free PMC article. - Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons.
Fjell J, Cummins TR, Dib-Hajj SD, Fried K, Black JA, Waxman SG. Fjell J, et al. Brain Res Mol Brain Res. 1999 Apr 20;67(2):267-82. doi: 10.1016/s0169-328x(99)00070-4. Brain Res Mol Brain Res. 1999. PMID: 10216225 - NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy.
Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG. Dib-Hajj SD, et al. Proc Natl Acad Sci U S A. 1998 Jul 21;95(15):8963-8. doi: 10.1073/pnas.95.15.8963. Proc Natl Acad Sci U S A. 1998. PMID: 9671787 Free PMC article. - NaN/Nav1.9: a sodium channel with unique properties.
Dib-Hajj S, Black JA, Cummins TR, Waxman SG. Dib-Hajj S, et al. Trends Neurosci. 2002 May;25(5):253-9. doi: 10.1016/s0166-2236(02)02150-1. Trends Neurosci. 2002. PMID: 11972962 Review.
Cited by
- Genetic Analysis of SCN11A, SCN10A, and SCN9A in Familial Episodic Pain Syndrome (FEPS) in Japan and Proposal of Clinical Diagnostic Criteria.
Noguchi A, Tezuka T, Okuda H, Kobayashi H, Harada KH, Yoshida T, Akioka S, Wada K, Takeya A, Kabata-Murasawa R, Kondo D, Ishikawa K, Asano T, Fujiwara M, Hishikawa N, Mizukami T, Hitomi T, Youssefian S, Nagai Y, Tanaka M, Eto K, Shiraishi H, Amaya F, Koizumi A, Takahashi T. Noguchi A, et al. Int J Mol Sci. 2024 Jun 21;25(13):6832. doi: 10.3390/ijms25136832. Int J Mol Sci. 2024. PMID: 38999942 Free PMC article. - Contactin associates with sodium channel Nav1.3 in native tissues and increases channel density at the cell surface.
Shah BS, Rush AM, Liu S, Tyrrell L, Black JA, Dib-Hajj SD, Waxman SG. Shah BS, et al. J Neurosci. 2004 Aug 18;24(33):7387-99. doi: 10.1523/JNEUROSCI.0322-04.2004. J Neurosci. 2004. PMID: 15317864 Free PMC article. - Infantile Pain Episodes Associated with Novel Nav1.9 Mutations in Familial Episodic Pain Syndrome in Japanese Families.
Okuda H, Noguchi A, Kobayashi H, Kondo D, Harada KH, Youssefian S, Shioi H, Kabata R, Domon Y, Kubota K, Kitano Y, Takayama Y, Hitomi T, Ohno K, Saito Y, Asano T, Tominaga M, Takahashi T, Koizumi A. Okuda H, et al. PLoS One. 2016 May 25;11(5):e0154827. doi: 10.1371/journal.pone.0154827. eCollection 2016. PLoS One. 2016. PMID: 27224030 Free PMC article. Clinical Trial. - Sensitization of TRPV1 receptors by TNF-α orchestrates the development of vincristine-induced pain.
Wang Y, Feng C, He H, He J, Wang J, Li X, Wang S, Li W, Hou J, Liu T, Fang D, Xie SQ. Wang Y, et al. Oncol Lett. 2018 Apr;15(4):5013-5019. doi: 10.3892/ol.2018.7986. Epub 2018 Feb 7. Oncol Lett. 2018. PMID: 29552137 Free PMC article. - Reversal of neuropathic pain in diabetes by targeting glycosylation of Ca(V)3.2 T-type calcium channels.
Orestes P, Osuru HP, McIntire WE, Jacus MO, Salajegheh R, Jagodic MM, Choe W, Lee J, Lee SS, Rose KE, Poiro N, Digruccio MR, Krishnan K, Covey DF, Lee JH, Barrett PQ, Jevtovic-Todorovic V, Todorovic SM. Orestes P, et al. Diabetes. 2013 Nov;62(11):3828-38. doi: 10.2337/db13-0813. Epub 2013 Jul 8. Diabetes. 2013. PMID: 23835327 Free PMC article.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical