Furin is involved in baculovirus envelope fusion protein activation - PubMed (original) (raw)
Furin is involved in baculovirus envelope fusion protein activation
Marcel Westenberg et al. J Virol. 2002 Jan.
Abstract
The Spodoptera exigua multicapsid nucleopolyhedrovirus (SeMNPV) Se8 gene was recently shown to encode the viral envelope fusion (F) protein. A 60-kDa C-terminal subunit (F1) of the 76-kDa primary translation product of this gene was found to be the major envelope protein of SeMNPV budded virus (BV) (W. F. J. IJkel, M. Westenberg, R. W. Goldbach, G. W. Blissard, J. M. Vlak, and D. Zuidema, Virology 275:30-41, 2000). A specific inhibitor was used to show that furin is involved in cleavage of the precursor envelope fusion (F0) protein. BV produced in the presence of the inhibitor possesses the uncleaved F0 protein, while an F protein with a mutation in the furin cleavage site was translocated to the plasma membrane but lost its fusogenic activity. These results indicate that cleavage of F0 is required to activate the SeMNPV F protein and is necessary for BV infectivity. Specific antibodies against F1 and against the putative N terminus (F2) of the primary translation product were used to show that the F protein is BV specific and that BVs contain both the 60- (F1) and 21-kDa (F2) cleavage products. In nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis both subunits migrate as a single 80-kDa protein, indicating that the subunits remain associated by a disulfide linkage. In addition, the presence of the F protein predominantly as a monomer suggests that disulfide links are not involved in oligomerization. Thus, the envelope fusion protein from group II nucleopolyhedroviruses of baculoviruses has properties similar to those of proteins from a number of vertebrate viruses.
Figures
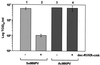
FIG. 1.
Effect of furin inhibitor dec-RVKR-cmk on Se_M_NPV and Ac_M_NPV BV infectivity. Se301 cells were infected with Se_M_NPV (lanes 1 and 2) or Ac_M_NPV (lanes 3 and 4) in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of furin inhibitor dec-RVKR-cmk. At 48 h p.i. the titer of progeny infectious BV in the cell culture supernatants was determined by TCID50 assays. The data presented are means and standard deviations of three independent experiments.
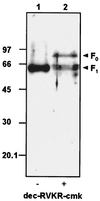
FIG. 2.
Western blot analysis of proteins obtained from Se_M_NPV BV, produced in the absence (−; lane 1) or presence (+ lane 2) of furin inhibitor dec-RVKR-cmk. BVs were incubated for 10 min at 95°C in Laemmli buffer and subjected to SDS-PAGE (12% polyacrylamide). Proteins were transferred onto an Immobilon-P membrane (Millipore), incubated with α-F1, and detected with a chemiluminescent substrate. Size standards in kilodaltons are on the left. F0, precursor Se_M_NPV F protein; F1, 60-kDa subunit of Se_M_NPV F protein.
FIG. 3.
Se_M_NPV-mediated fusion of Se301 cells. Cells were infected with Se_M_NPV in the absence (A) or presence (B) of furin inhibitor dec-RVKR-cmk. Forty-eight hours p.i. cells were incubated for 2 min with medium, pH 5.0. Syncytium formation was scored 4 h after dropping the pH by phase-contrast microscopy.
FIG. 4.
Localization studies with Se_M_NPV wild-type F and mutant FK149-GFP fusion proteins (A to C) and their ability to mediate cell-to-cell fusion in Sf21 cells (D to F). For the localization studies, Sf21 cells were transfected with plasmid p166AcV5-Se8GFP (A), p166AcV5-Se8(K149)GFP (B), or control plasmid p166AcV5-GFP (C). Fluorescence was examined 48 h after transfection by confocal laser scanning microscopy. For pH-dependent membrane fusion, Sf21 cells were transfected with plasmid p166AcV5-Se8 (D), p166AcV5-Se8(K149) (E), or control p166BRNX-AcV5 (F). Forty-eight hours after transfection, cells were treated for 2 min with medium, pH 5.0. Four hours after the pH drop syncytium formation was scored by phase-contrast microscopy.
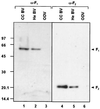
FIG. 5.
Western blot analysis of reduced proteins of Se_M_NPV virus preparations. Cell culture-derived (CC) BVs, hemolymph-derived (He) BVs, and polyhedra-derived ODVs were incubated for 10 min at 95°C in Laemmli buffer and subjected to SDS-PAGE (12% polyacrylamide). Proteins were transferred onto an Immobilon-P membrane (Millipore), incubated with either α-F1 (left) or α-F2 (right) antibodies, and detected with a chemiluminescent substrate. Size standards in kilodaltons are on the left. F1, 60-kDa subunit of Se_M_NPV F protein; F2, 21-kDa subunit of Se_M_NPV F protein.
FIG. 6.
Western blot analysis of nonreduced proteins of Se_M_NPV BV. Cell culture-derived BVs were incubated for 10 min at 95°C in the presence of 37.5 mM iodoacetamide and in the absence of 2-mercaptoethanol and electrophoresed in a 12% (A) or 6% (B) SDS-polyacrylamide gel. Proteins were transferred onto an Immobilon-P membrane (Millipore), incubated with either α-F1 or α-F2, and detected with a chemiluminescent substrate. Size standards (kilodaltons) are indicated. F1,2, 60- and 21-kDa disulfide-linked subunits of Se_M_NPV F protein.
Similar articles
- A novel baculovirus envelope fusion protein with a proprotein convertase cleavage site.
IJkel WF, Westenberg M, Goldbach RW, Blissard GW, Vlak JM, Zuidema D. IJkel WF, et al. Virology. 2000 Sep 15;275(1):30-41. doi: 10.1006/viro.2000.0483. Virology. 2000. PMID: 11017785 - Pseudotyping Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV): F proteins from group II NPVs are functionally analogous to AcMNPV GP64.
Lung O, Westenberg M, Vlak JM, Zuidema D, Blissard GW. Lung O, et al. J Virol. 2002 Jun;76(11):5729-36. doi: 10.1128/jvi.76.11.5729-5736.2002. J Virol. 2002. PMID: 11992001 Free PMC article. - GP64 of group I nucleopolyhedroviruses cannot readily rescue infectivity of group II f-null nucleopolyhedroviruses.
Westenberg M, Vlak JM. Westenberg M, et al. J Gen Virol. 2008 Feb;89(Pt 2):424-431. doi: 10.1099/vir.0.83342-0. J Gen Virol. 2008. PMID: 18198373 - Secretion of the respiratory syncytial virus fusion protein from insect cells using the baculovirus expression system.
Tan BH, Brown G, Sugrue RJ. Tan BH, et al. Methods Mol Biol. 2007;379:149-61. doi: 10.1007/978-1-59745-393-6_11. Methods Mol Biol. 2007. PMID: 17502677 Review. - Determinants of organ tropism of sendai virus.
Tashiro M, McQueen NL, Seto JT. Tashiro M, et al. Front Biosci. 1999 Oct 1;4:D642-5. doi: 10.2741/tashiro. Front Biosci. 1999. PMID: 10502551 Review.
Cited by
- Display of heterologous proteins on gp64null baculovirus virions and enhanced budding mediated by a vesicular stomatitis virus G-stem construct.
Zhou J, Blissard GW. Zhou J, et al. J Virol. 2008 Feb;82(3):1368-77. doi: 10.1128/JVI.02007-07. Epub 2007 Nov 7. J Virol. 2008. PMID: 17989172 Free PMC article. - Unraveling the entry mechanism of baculoviruses and its evolutionary implications.
Wang M, Wang J, Yin F, Tan Y, Deng F, Chen X, Jehle JA, Vlak JM, Hu Z, Wang H. Wang M, et al. J Virol. 2014 Feb;88(4):2301-11. doi: 10.1128/JVI.03204-13. Epub 2013 Dec 11. J Virol. 2014. PMID: 24335309 Free PMC article. - Mutagenesis and nuclear magnetic resonance analyses of the fusion peptide of Helicoverpa armigera single nucleocapsid nucleopolyhedrovirus F protein.
Tan Y, Jiang L, Wang M, Yin F, Deng F, Liu M, Hu Z, Wang H. Tan Y, et al. J Virol. 2008 Aug;82(16):8138-48. doi: 10.1128/JVI.00368-08. Epub 2008 Jun 4. J Virol. 2008. PMID: 18524820 Free PMC article. - Determination of the protein composition of the occlusion-derived virus of Autographa californica nucleopolyhedrovirus.
Braunagel SC, Russell WK, Rosas-Acosta G, Russell DH, Summers MD. Braunagel SC, et al. Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):9797-802. doi: 10.1073/pnas.1733972100. Epub 2003 Aug 6. Proc Natl Acad Sci U S A. 2003. PMID: 12904572 Free PMC article. - A cellular Drosophila melanogaster protein with similarity to baculovirus F envelope fusion proteins.
Lung O, Blissard GW. Lung O, et al. J Virol. 2005 Jul;79(13):7979-89. doi: 10.1128/JVI.79.13.7979-7989.2005. J Virol. 2005. PMID: 15956544 Free PMC article.
References
- Adams, J. R., and J. T. McClintock. 1991. Nuclear polyhedrosis viruses of insects, p. 87–204. In J. R. Adams and J. R. Bonami (ed.), Atlas of invertebrate viruses. CRC Press, Boca Raton, Fla.
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1994. Current protocols in molecular biology. Greene Publishing Associates and Wiley Interscience, New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous