Biogenesis of T pili in Agrobacterium tumefaciens requires precise VirB2 propilin cleavage and cyclization - PubMed (original) (raw)
Biogenesis of T pili in Agrobacterium tumefaciens requires precise VirB2 propilin cleavage and cyclization
Erh-Min Lai et al. J Bacteriol. 2002 Jan.
Abstract
VirB2 propilin is processed by the removal of a 47-amino-acid signal peptide to generate a 74-amino-acid peptide product in both Escherichia coli and Agrobacterium tumefaciens. The cleaved VirB2 protein is further cyclized to form the T pilin in A. tumefaciens but not in E. coli. Mutations in the signal peptidase cleavage sequence of VirB2 propilin cause the formation of aberrant T pilin and also severely attenuate virulence. No T pilus was observed in these mutants. The potential role of the exact VirB2 propilin cleavage and cyclization in T pilus biogenesis and virulence is discussed.
Figures
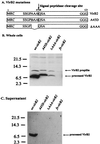
FIG. 1.
Cyclization of T pilin occurs in A. tumefaciens but not in E. coli. (A) MALDI-TOF MS data derived from sample preparations with whole cells of E. coli DH5α expressing VirB2 (pUCD4805) versus A. tumefaciens NT1REB expressing VirB2 (pUCD4813) in the absence of its Ti plasmid (1). Purified T pili and vectors served as controls. The signal at m/z 7,202.2 corresponds to linear processed VirB2, while the signal at m/z 7,184.3 corresponds to T pilin (indicated by arrows). (B) Immunoblot of linear VirB2 and T pilin. Whole-cell lysates from E. coli DH5α containing either pTTQ18 (vector) or pUCD4805 (VirB2) or from A. tumefaciens NT1REB containing either pUCD4813 (VirB2, no Ti) or pTiC58 were fractionated by Tricine-SDS-16.5% PAGE and analyzed by immunoblotting using anti-VirB2 antibody. Molecular masses of protein size markers (in kilodaltons) are indicated on the left. Positions of linear processed VirB2 and T pilin are marked by arrows.
FIG. 2.
Processing and exportation analyses of VirB2 mutant proteins in A. tumefaciens. (A) Mutations in the signal peptidase cleavage sequences of VirB2. Proteins are drawn as boxes, the first and last amino acid residues are numbered, and the signal peptidase cleavage site A47-Q48 is indicated. The A45D mutant carries a substitution mutation that replaces Ala-45 (A). with Asp (D) (in bold), and the ΔAAA mutation is a deletion of three Ala residues at positions 45 to 47. (B and C) Strain NT1RE containing either pJK270 (wild type; wt-virB2), pUCD4605 (A45D-virB2), pUCD4606 (Δ_AAA-virB2_), or pUCD4607 (frameshift mutant, fs-virB2) was induced with acetosyringone in I medium at 19°C for 72 h (6). Proteins from whole-cell lysates (B) and from their concentrated supernatants (C) were fractionated by Tricine-SDS-16.5% PAGE and immunoblotted with anti-VirB2 antibody. Molecular masses of protein markers are shown on the left side of each immunoblot. Unprocessed and processed VirB2 proteins are indicated.
FIG. 3.
Localization of mutant and wild-type VirB2 proteins in A. tumefaciens NT1RE. Strain NT1RE contained either pJK270 (wild-type virB2) (lanes 1 and 2), pUCD4605 (A45D-virB2) (lanes 3 and 4), pUCD4606 (ΔAAA-virB2) (lanes 5 and 6), or pUCD4607 (frameshift mutation virB2) (lanes 7 and 8). Isolated membrane vesicles from each strain were treated with 2% Sarkosyl to separate inner membrane (lanes 1, 3, 5, and 7)- and outer membrane (lanes 2, 4, 6, and 8)-associated proteins. Proteins were fractionated by Tricine-SDS-16.5% PAGE and analyzed by immunoblotting with anti-VirB2 antibody. Unprocessed and processed VirB2 proteins are indicated.
FIG. 4.
Tumorigenicity assay on D. stramonium. Representative sections of the stem of each test plant inoculated with the indicated strain are shown at 4 to 5 weeks postinoculation. The inoculated strains of A. tumefaciens NT1RE contained either pJK270 (wild-type virB2 [wt-virB2]), pUCD4605 (A45D-virB2), pUCD4606 (ΔAAA-virB2), or pUCD4607 (virB2 frameshift mutant [fs-virB2]).
Similar articles
- VirB2 is a processed pilin-like protein encoded by the Agrobacterium tumefaciens Ti plasmid.
Jones AL, Lai EM, Shirasu K, Kado CI. Jones AL, et al. J Bacteriol. 1996 Oct;178(19):5706-11. doi: 10.1128/jb.178.19.5706-5711.1996. J Bacteriol. 1996. PMID: 8824616 Free PMC article. - Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens.
Lai EM, Kado CI. Lai EM, et al. J Bacteriol. 1998 May;180(10):2711-7. doi: 10.1128/JB.180.10.2711-2717.1998. J Bacteriol. 1998. PMID: 9573157 Free PMC article. - Membrane location of the Ti plasmid VirB proteins involved in the biosynthesis of a pilin-like conjugative structure on Agrobacterium tumefaciens.
Shirasu K, Kado CI. Shirasu K, et al. FEMS Microbiol Lett. 1993 Aug 1;111(2-3):287-94. doi: 10.1111/j.1574-6968.1993.tb06400.x. FEMS Microbiol Lett. 1993. PMID: 8405938 - Promiscuous DNA transfer system of Agrobacterium tumefaciens: role of the virB operon in sex pilus assembly and synthesis.
Kado CI. Kado CI. Mol Microbiol. 1994 Apr;12(1):17-22. doi: 10.1111/j.1365-2958.1994.tb00990.x. Mol Microbiol. 1994. PMID: 7914664 Review. - The T-pilus of Agrobacterium tumefaciens.
Lai EM, Kado CI. Lai EM, et al. Trends Microbiol. 2000 Aug;8(8):361-9. doi: 10.1016/s0966-842x(00)01802-3. Trends Microbiol. 2000. PMID: 10920395 Review.
Cited by
- Mutations Suppressing the Lack of Prepilin Peptidase Provide Insights Into the Maturation of the Major Pilin Protein in Cyanobacteria.
Linhartová M, Skotnicová P, Hakkila K, Tichý M, Komenda J, Knoppová J, Gilabert JF, Guallar V, Tyystjärvi T, Sobotka R. Linhartová M, et al. Front Microbiol. 2021 Oct 12;12:756912. doi: 10.3389/fmicb.2021.756912. eCollection 2021. Front Microbiol. 2021. PMID: 34712217 Free PMC article. - Secretion of Pertussis Toxin from Bordetella pertussis.
Burns DL. Burns DL. Toxins (Basel). 2021 Aug 18;13(8):574. doi: 10.3390/toxins13080574. Toxins (Basel). 2021. PMID: 34437445 Free PMC article. Review. - Arabidopsis RETICULON-LIKE4 (RTNLB4) Protein Participates in Agrobacterium Infection and VirB2 Peptide-Induced Plant Defense Response.
Huang FC, Hwang HH. Huang FC, et al. Int J Mol Sci. 2020 Mar 3;21(5):1722. doi: 10.3390/ijms21051722. Int J Mol Sci. 2020. PMID: 32138311 Free PMC article. - Agrobacterium-mediated plant transformation: biology and applications.
Hwang HH, Yu M, Lai EM. Hwang HH, et al. Arabidopsis Book. 2017 Oct 20;15:e0186. doi: 10.1199/tab.0186. eCollection 2017. Arabidopsis Book. 2017. PMID: 31068763 Free PMC article. - Function and Regulation of Agrobacterium tumefaciens Cell Surface Structures that Promote Attachment.
Thompson MA, Onyeziri MC, Fuqua C. Thompson MA, et al. Curr Top Microbiol Immunol. 2018;418:143-184. doi: 10.1007/82_2018_96. Curr Top Microbiol Immunol. 2018. PMID: 29998422 Free PMC article. Review.
References
- Eisenbrandt, R., M. Kalkum, E. M. Lai, R. Lurz, C. I. Kado, and E. Lanka. 1999. Conjugative pili of IncP plasmids and the Ti plasmid T pilus are composed of cyclic subunits. J. Biol. Chem. 274:22548–22555. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources