Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes - PubMed (original) (raw)
Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes
R Madrid et al. EMBO J. 2001.
Abstract
Aquaporin 4 (AQP4) is the predominant water channel in the brain. It is targeted to specific membrane domains of astrocytes and plays a crucial role in cerebral water balance in response to brain edema formation. AQP4 is also specifically expressed in the basolateral membranes of epithelial cells. However, the molecular mechanisms involved in its polarized targeting and membrane trafficking remain largely unknown. Here, we show that two independent C-terminal signals determine AQP4 basolateral membrane targeting in epithelial MDCK cells. One signal involves a tyrosine-based motif; the other is encoded by a di-leucine-like motif. We found that the tyrosine-based basolateral sorting signal also determines AQP4 clathrin-dependent endocytosis through direct interaction with the mu subunit of AP2 adaptor complex. Once endocytosed, a regulated switch in mu subunit interaction changes AP2 adaptor association to AP3. We found that the stress-induced kinase casein kinase (CK)II phosphorylates the Ser276 immediately preceding the tyrosine motif, increasing AQP4-mu 3A interaction and enhancing AQP4-lysosomal targeting and degradation. AQP4 phosphorylation by CKII may thus provide a mechanism that regulates AQP4 cell surface expression.
Figures
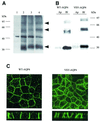
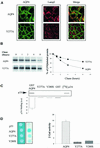
Fig. 1. Western blot and immunolocalization analysis of AQP4 in MDCK cells. (A) Western blot probed with anti-AQP4 antibody. Lane 1: control MDCK cells, lane 2: wild-type AQP4, lane 3: VSV-AQP4 transfected MDCK cells. Glycopeptidase F treatment did not affect VSV-AQP4 MW indicating the protein was not glycosylated (lane 4). Arrowheads indicate 34, 60, 97 kDa bands specifically detected. (B) Steady-state repartition of AQP4 in apical and basolateral membranes. Western blot of plasma membrane fraction of WT and VSV-AQP4 cells biotinylated from the basolateral (Bl) and the apical (Ap) side growing on a permeable support and probed with anti-AQP4 antibody. (C) Confocal images of WT and VSV-AQP4 protein stably transfected in MDCK cells and detected using anti-AQP4 and VSV antibodies, respectively. The upper and lower images show focal planes parallel and perpendicular to the epithelium, respectively.
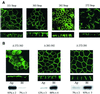
Fig. 2. Two independent motifs determine AQP4 basolateral targeting. (A) Confocal microscopy images of VSV-AQP4 mutants missing the 3 (321 Stop), 21 (303 Stop), 42 (282 Stop) and 52 (272 Stop) C-terminal amino acids, respectively. (B) Confocal microscopy images of AQP4-Δ272–302, Δ272–281 and Δ282–302 mutants bearing internal deletion of 31, 10 and 21 amino acid residues, respectively. An example of quantification of apical versus basolateral expression of the mutants by specific membrane biotinylation and western blotting (see Materials and methods) is shown together with the mean ± SD of at least three experiments.
Fig. 3. The proximal basolateral sorting signal juxtaposes a tyrosine motif. Confocal microscopy images of VSV-AQP4 mutants bearing Y277A and V280S point mutations in the tyrosine motif (GSYMEV) (A) or GSY-AAA and G,Y-A.A and SY-A.A substitutions (B). All these mutations were introduced in the backbone construct AQP4-Δ282–302 in which the second basolateral sorting signal was deleted. Two different focal planes taken at the level of the apical and basolateral membranes of the same cells are shown in (A). Apical versus basolateral repartition was quantified by specific membrane biotinylation and western blotting (see legend to Figure 2).
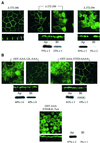
Fig. 4. Di-leucine-like and acidic cluster constitute the second basolateral sorting signal. (A) Confocal microscopy images of VSV-AQP4 mutants bearing increasing deletions of DNRSQ, VETE and LIL in the DNRSQVETEDLILKPGVVHVI sequence (Δ272–286, Δ272–290 and Δ272–294, respectively). (B) Simultaneous (ETEDLIL-7×A) but not serial alanine substitution of the acidic cluster (ETED-AAA) and the leucine like motif (LIL-AAA) was necessary to target AQP4 to the apical membranes. Two different focal planes taken at the level of the apical and basolateral membranes of the same cells are shown in (A) and (B). In mutants shown in (A) and (B), the tyrosine-based basolateral sorting motif was removed by deletion (Δ272–281) and GSY-AAA substitution, respectively. Apical versus basolateral repartition was quantified by biotinylation and western blotting (see legend to Figure 2).
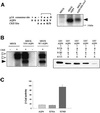
Fig. 5. AQP4 is endocytosed by clathrin-dependent mechanisms and interacts with the µ subunit of AP2 complex. (A) Western blot probed with anti-AQP4 antibody illustrating AQP4 endocytosis assay in MDCK cells assessed by biotinylation experiments. AQP4 was biotinylated at 4°C (_t_0) and allowed to internalize for 15 min at 37°C (_t_15). Endocytosed AQP4 was protected from glutathione reduction (+), isolated using streptavidin beads and quantified by western blotting using anti-AQP4. (B) Percentage of endocytosis measured for VSV-AQP4 in control and hyperosmotic conditions (AQP4 and Sucrose, respectively), Y277A and V280S mutants. Data represent the means ± SD of at least four experiments. * indicates significant difference against the control p<0.05 (paired Student’s _t_-test). (C) Autoradiography of in vitro translated 35S-labeled µ2 subunit retained by in vitro binding assay using 3 µg of control GST, GST–AQP4-Cter, GST–AQP4-Y277A (Y277A) and GST–AQP4-V280S (V280S) fusion proteins. One-fifth of the µ2 input used in the binding assay is shown in the right-hand lane ([35S]µ2). Results are quantified in the histogram. Values on the ordinate are arbitrary units; the reference (GST) was assigned a value of 1. Assays were performed in triplicate. (D) Two-hybrid assay of AQP4–µ2 interaction. EGY48 yeast strain co-transformed with pACTII-µ2 and wild-type or mutants LEXA-AQP4 baits (AQP4, Y277A or V280S, respectively) were patched on selective medium, replica plated on Whatmann filters and tested for interaction using β-galactosidase activity (blue spots on right-hand image). LEXA-p53 fusion protein (p53) was used as a negative control. Right panel; the strength of interaction was quantified from β-gal activity measured in liquid culture. Values are expressed as means in arbitrary units/min/cell ± SD (n = 4) from three different yeast transformants.
Fig. 6. AQP4 is targeted to lysosomes and interacts with µ3A. (A) Confocal microscopy images of wild-type VSV-AQP4 (AQP4, top images) and VSV-AQP4-Y277A (Y277A, bottom images) labeled with anti-VSV antibody (green) and anti-LampI antibody (red). Co-localization is shown as yellow staining in the merged right-hand images. (B) Left panel: autoradiography of wild-type VSV-AQP4 and AQP4-Y277A mutant proteins pulse-labeled with [35S]methionine, chased for 0–8 h and immunoprecipitated using anti-VSV antibody. Right panel: radioactivity of the bands was quantified and the results are expressed in percent of the radioactivity measured at _t_0. Data are the means ± SD of at least three experiments. (C) Autoradiography of in vitro translated 35S-labeled µ3A subunit retained by in vitro binding assay using 3 µg of control GST, GST–AQP4-Cter, GST–AQP4-Y277A and V280S mutant fusion proteins. The quantification shown on the histogram was performed according to Figure 5C. (D) Two-hybrid assay of AQP4–µ3A interaction. Left panel; EGY48 yeast strain co-transformed with pACTII-µ3A and wild-type or mutant LEXA-AQP4 baits (AQP4, Y277A or V280S, respectively) were patched on selective medium, replica plated on Whatmann filters and tested for interaction using β-galactosidase activity (blue spots on right-hand image). LEXA-p53 fusion protein (p53) was used as a negative control. Right panel: the strength of interaction was quantified from β-gal activity measured in liquid culture. Values are expressed as means in arbitrary units/min/cell ± SD (n = 5) from three different yeast transformants.
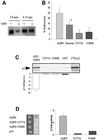
Fig. 7. CKII phosphorylates AQP4 and modulates AQP4–µ3A interaction. (A) Left panel: sequence alignment of tyrosine-based motif favored by µ3A, amino acid sequence surrounding the AQP4 tyrosine-based motif and CKII consensus site. Right panel: autoradiography of wild-type AQP4 and AQP4-SSS-AAA mutant immunoprecipitated using the anti-VSV antibody from cells labeled with [32P]ortho phosphate and exhibiting similar AQP4-expression level. A 32P-labeled AQP4 (arrowhead) was immunoprecipitated from VSV-AQP4 transfected cells (MDCK-AQP4) but not from control MDCK cells. (B) In vitro phosphorylation of AQP4 and GST–AQP4-Cter by CKII using γ-[32P]ATP. Left panel: AQP4 protein immunoprecipitated from VSV-AQP4-MDCK cells and control MDCK cells (using anti-VSV antibody) and WT-AQP4-MDCK cells (using anti-AQP4 antibody) were incubated with (+) or without (–) CKII in the presence of [32P]γ-ATP. Open and filled arrowheads indicate autophosphorylated CKII subunits and 32P-labeled AQP4, respectively. Right panel: GST–AQP4-Cter fusion proteins containing, either wild-type AQP4 (left lane) or mutant AQP4 sequences in which only one of the three potential CKII sites (Ser275, Ser285 and Ser315) was conserved, were phosphorylated by CKII in the presence of [32P]γ-ATP. GST–AQP4-Cter fusion protein in which the three serines were replaced by alanines exhibited background level of phosphorylation (last lane). (C) Strength of interaction measured in the yeast two-hybrid assay between µ3A and wild-type AQP4 (AQP4) and the mutant baits S276A and S276D. Values are expressed means ± SD (n = at least five in each group) in arbitrary units/min/cell.
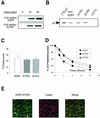
Fig. 8. CKII phosphorylation increases AQP4 lysosomal targeting and degradation but not endocytosis. (A) Cell surface delivery of VSV-AQP4 and S276D mutant revealed in pulse–chase experiments coupled to cell surface biotinylation. Both newly synthesized proteins were detected on the cell surface after a 30 min chase period. (B) Autoradiography of in vitro translated 35S-labeled µ2 subunit retained on 3 µg of wild-type GST–AQP4-Cter and the mutant constructs S276A and S276D. GST–AQP4-Y277A was used as a negative control (last lane). One fifth of the µ2 translation reaction used in the assay is shown in the left-hand lane ([35S]µ2). (C) Percentage of endocytosis of AQP4 measured in wild-type AQP4, AQP4-S276D and AQP4-S276A-MDCK cells using the biotinylation protocol. Values are expressed means ± SD (n = at least three in each group). (D) Degradation rate measured in pulse–chase experiments for wild-type AQP4, S276D and S276A mutants. Results are expressed as a percentage of the radioactivity measured at _t_0 (see Figure 5). (E) Confocal images of AQP4-S276D-MDCK cells processed using anti-VSV (green) and anti-LampI antibody (red). Co-localization is shown as yellow staining in merged right-hand image. Arrowheads point to visible AQP4 basolateral expression.
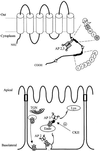
Fig. 9. Schematic illustration of AQP4 sorting motifs and trafficking in epithelial cells. Upper panel: hydropathy analysis of AQP4 sequence predicted six transmembrane domains connected by five loops. The two C-terminal basolateral targeting motifs (GSYMEV and ETEDLIL) are shown. Stars indicate the three serines phosphorylated by CKII. The adaptor complexes (AP2 and AP3) interacting with the tyrosine-based motif are shown. The residues critical for AQP4–µ subunits interactions (Y277 and V280) are shaded. Lower panel: model of AQP4 trafficking in epithelial cells. _Trans_-Golgi network (TGN), endosomal (Endo) and lysosomal (Lys) compartments are shown.
Similar articles
- In vitro binding of clathrin adaptors to sorting signals correlates with endocytosis and basolateral sorting.
Heilker R, Manning-Krieg U, Zuber JF, Spiess M. Heilker R, et al. EMBO J. 1996 Jun 3;15(11):2893-9. EMBO J. 1996. PMID: 8654387 Free PMC article. - Basolateral sorting of human poliovirus receptor alpha involves an interaction with the mu1B subunit of the clathrin adaptor complex in polarized epithelial cells.
Ohka S, Ohno H, Tohyama K, Nomoto A. Ohka S, et al. Biochem Biophys Res Commun. 2001 Oct 5;287(4):941-8. doi: 10.1006/bbrc.2001.5660. Biochem Biophys Res Commun. 2001. PMID: 11573956 - Identification of a novel di-leucine motif mediating K(+)/Cl(-) cotransporter KCC2 constitutive endocytosis.
Zhao B, Wong AY, Murshid A, Bowie D, Presley JF, Bedford FK. Zhao B, et al. Cell Signal. 2008 Oct;20(10):1769-79. doi: 10.1016/j.cellsig.2008.06.011. Epub 2008 Jun 24. Cell Signal. 2008. PMID: 18625303 - Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport.
Amiry-Moghaddam M, Frydenlund DS, Ottersen OP. Amiry-Moghaddam M, et al. Neuroscience. 2004;129(4):999-1010. doi: 10.1016/j.neuroscience.2004.08.049. Neuroscience. 2004. PMID: 15561415 Review. - Trafficking of lysosomal membrane proteins in polarized kidney cells.
Hunziker W, Simmen T, Höning S. Hunziker W, et al. Nephrologie. 1996;17(7):347-50. Nephrologie. 1996. PMID: 8987042 Review.
Cited by
- Brain expression of the water channels aquaporin-1 and -4 in mice with acute liver injury, hyperammonemia and brain edema.
Eefsen M, Jelnes P, Schmidt LE, Vainer B, Bisgaard HC, Larsen FS. Eefsen M, et al. Metab Brain Dis. 2010 Sep;25(3):315-23. doi: 10.1007/s11011-010-9213-y. Epub 2010 Oct 12. Metab Brain Dis. 2010. PMID: 20938728 - Phosphorylation-Dependent Regulation of Mammalian Aquaporins.
Nesverova V, Törnroth-Horsefield S. Nesverova V, et al. Cells. 2019 Jan 23;8(2):82. doi: 10.3390/cells8020082. Cells. 2019. PMID: 30678081 Free PMC article. Review. - Basolateral sorting signals regulating tissue-specific polarity of heteromeric monocarboxylate transporters in epithelia.
Castorino JJ, Deborde S, Deora A, Schreiner R, Gallagher-Colombo SM, Rodriguez-Boulan E, Philp NJ. Castorino JJ, et al. Traffic. 2011 Apr;12(4):483-98. doi: 10.1111/j.1600-0854.2010.01155.x. Epub 2011 Feb 1. Traffic. 2011. PMID: 21199217 Free PMC article. - Mechanisms of cell polarity and aquaporin sorting in the nephron.
Edemir B, Pavenstädt H, Schlatter E, Weide T. Edemir B, et al. Pflugers Arch. 2011 Jun;461(6):607-21. doi: 10.1007/s00424-011-0928-3. Epub 2011 Feb 16. Pflugers Arch. 2011. PMID: 21327781 Review. - Astrocyte Aquaporin Dynamics in Health and Disease.
Potokar M, Jorgačevski J, Zorec R. Potokar M, et al. Int J Mol Sci. 2016 Jul 13;17(7):1121. doi: 10.3390/ijms17071121. Int J Mol Sci. 2016. PMID: 27420057 Free PMC article. Review.
References
- Brown D. and Breton,S. (2000) Sorting proteins to their target membranes. Kidney Int., 57, 816–824. - PubMed
- Brown D., Weyer,P. and Orci,L. (1988) Vasopressin stimulates endocytosis in kidney collecting duct principal cells. Eur. J. Cell Biol., 46, 336–341. - PubMed
- Deen P.M. and van Os,C.H. (1998) Epithelial aquaporins. Curr. Opin. Cell Biol., 10, 435–442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials