Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus - PubMed (original) (raw)
doi: 10.1086/338710. Epub 2001 Dec 17.
David N Bowser, Leanne M Dibbens, Rita Singh, Fiona Phillips, Robyn H Wallace, Michaella C Richards, David A Williams, John C Mulley, Samuel F Berkovic, Ingrid E Scheffer, Steven Petrou
Affiliations
- PMID: 11748509
- PMCID: PMC384926
- DOI: 10.1086/338710
Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus
Louise A Harkin et al. Am J Hum Genet. 2002 Feb.
Abstract
Recent findings from studies of two families have shown that mutations in the GABA(A)-receptor gamma2 subunit are associated with generalized epilepsies and febrile seizures. Here we describe a family that has generalized epilepsy with febrile seizures plus (GEFS(+)), including an individual with severe myoclonic epilepsy of infancy, in whom a third GABA(A)-receptor gamma2-subunit mutation was found. This mutation lies in the intracellular loop between the third and fourth transmembrane domains of the GABA(A)-receptor gamma2 subunit and introduces a premature stop codon at Q351 in the mature protein. GABA sensitivity in Xenopus laevis oocytes expressing the mutant gamma2(Q351X) subunit is completely abolished, and fluorescent-microscopy studies have shown that receptors containing GFP-labeled gamma2(Q351X) protein are retained in the lumen of the endoplasmic reticulum. This finding reinforces the involvement of GABA(A) receptors in epilepsy.
Figures
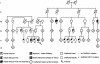
Figure 1
Pedigree of an Australian family with GEFS+. An asterisk (*) indicates members carrying the Q351X mutation in GABRG2, and a minus sign (−) indicates members who were tested and are negative for the Q351X mutation.
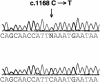
Figure 2
Sequencing trace of a portion of exon 9 GABRG2, showing the c.1168C→T transition (arrow). The upper chromatogram shows the mutation, and the lower chromatogram shows the control sequence.
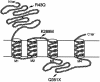
Figure 3
Schematic representation of the GABRG2 protein, with arrows indicating the positions of mutations associated with epilepsy.
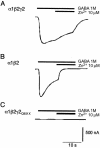
Figure 4
Two-electrode voltage-clamp recordings in oocytes, demonstrating that coexpression of the GABRG2Q351X abolishes the response to GABA. For all recordings,
_n_=6
. The current and time scale bars apply to all traces. Holding potential was −80 mV. A, Injection of oocytes with wild-type GABRA1:GABRB2:GABRG2 cRNAs (1:1:10 ratio, to favor assembly of γ subunit–containing complexes), resulting in both a robust inward current response to GABA and a low sensitivity to Zn2+ blockade, characteristic of GABRG2 coexpression. B, Expression of GABRA1:GABRB2 only (1:1 ratio). This also produced robust responses to GABA, but with high sensitivity to blockade by Zn2+. C, Expression of GABRA1:GABRB2:GABRG2Q351X subunits (1:1:10 ratio), abolishing GABA-induced currents.
Figure 5
Imaging of GFP-tagged GABRG2 expressed with GABRA1 and GABRB2 subunits, revealing the fate of GABRG2Q351X-containing receptors in HEK293 cells. Images were obtained by confocal fluorescence microscopy (excitation, 488 nm; emission, >515 nm). The scale bar in panel C corresponds to 5 microns and applies to all three panels. A, EGFP-tagged wild-type GABRG2 subunit, found in both the membrane and the intracellular compartment (
_n_=20
individual cells analyzed). B, EGFP-tagged GABRG2Q351X, found only in the intracellular compartment (
_n_=20
individual cells analyzed). C, ER-targeted EYFP expression, demonstrating subcellular distribution of ER that is remarkably similar to that seen in B.
Similar articles
- First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene.
Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud'homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E. Baulac S, et al. Nat Genet. 2001 May;28(1):46-8. doi: 10.1038/ng0501-46. Nat Genet. 2001. PMID: 11326274 - A novel GABRG2 mutation, p.R136*, in a family with GEFS+ and extended phenotypes.
Johnston AJ, Kang JQ, Shen W, Pickrell WO, Cushion TD, Davies JS, Baer K, Mullins JGL, Hammond CL, Chung SK, Thomas RH, White C, Smith PEM, Macdonald RL, Rees MI. Johnston AJ, et al. Neurobiol Dis. 2014 Apr;64:131-141. doi: 10.1016/j.nbd.2013.12.013. Epub 2014 Jan 7. Neurobiol Dis. 2014. PMID: 24407264 Free PMC article. - The genetics of febrile seizures and related epilepsy syndromes.
Hirose S, Mohney RP, Okada M, Kaneko S, Mitsudome A. Hirose S, et al. Brain Dev. 2003 Aug;25(5):304-12. doi: 10.1016/s0387-7604(03)00026-3. Brain Dev. 2003. PMID: 12850508 Review. - The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression.
Kang JQ, Shen W, Macdonald RL. Kang JQ, et al. J Neurosci. 2009 Mar 4;29(9):2845-56. doi: 10.1523/JNEUROSCI.4772-08.2009. J Neurosci. 2009. PMID: 19261880 Free PMC article. - Myoclonic seizures in the context of generalized epilepsy with febrile seizures plus (GEFS+).
Baulac M, Gourfinkel-An I, Baulac S, Leguern E. Baulac M, et al. Adv Neurol. 2005;95:119-25. Adv Neurol. 2005. PMID: 15508917 Review. No abstract available.
Cited by
- GABRG2 mutations in genetic epilepsy with febrile seizures plus: structure, roles, and molecular genetics.
Li X, Guo S, Sun Y, Ding J, Chen C, Wu Y, Li P, Sun T, Wang X. Li X, et al. J Transl Med. 2024 Aug 14;22(1):767. doi: 10.1186/s12967-024-05387-1. J Transl Med. 2024. PMID: 39143639 Free PMC article. Review. - A novel genetic locus for juvenile myoclonic epilepsy at chromosome 5q12-q14.
Kapoor A, Ratnapriya R, Kuruttukulam G, Anand A. Kapoor A, et al. Hum Genet. 2007 Jul;121(6):655-62. doi: 10.1007/s00439-007-0360-0. Epub 2007 Apr 13. Hum Genet. 2007. PMID: 17431681 - Molecular pathology of genetic epilepsies associated with GABAA receptor subunit mutations.
Macdonald RL, Kang JQ. Macdonald RL, et al. Epilepsy Curr. 2009 Jan-Feb;9(1):18-23. doi: 10.1111/j.1535-7511.2008.01278.x. Epilepsy Curr. 2009. PMID: 19396344 Free PMC article. - Sodium channel SCN1A and epilepsy: mutations and mechanisms.
Escayg A, Goldin AL. Escayg A, et al. Epilepsia. 2010 Sep;51(9):1650-8. doi: 10.1111/j.1528-1167.2010.02640.x. Epilepsia. 2010. PMID: 20831750 Free PMC article. Review. - Differential molecular and behavioural alterations in mouse models of GABRG2 haploinsufficiency versus dominant negative mutations associated with human epilepsy.
Warner TA, Shen W, Huang X, Liu Z, Macdonald RL, Kang JQ. Warner TA, et al. Hum Mol Genet. 2016 Aug 1;25(15):3192-3207. doi: 10.1093/hmg/ddw168. Epub 2016 Jun 23. Hum Mol Genet. 2016. PMID: 27340224 Free PMC article.
References
Electronic-Database Information
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for human GABRG2 cDNA reference sequence [accession number nm_000816])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for GEFS+ [MIM <604233>]) - PubMed
References
- Alekov AK, Rahman MM, Mitrovic N, Lehmann-Horn F, Lerche H (2001) Enhanced inactivation and acceleration of activation of the sodium channel associated with epilepsy in man. Eur J Neurosci 13:2171–2176 - PubMed
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ (1998) International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50:291–313 - PubMed
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud’homme J-F, Baulac M, Brice A, Bruzzone R, LeGuern E (2001) First evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ2-subunit gene. Nat Genet 28:46–48 - PubMed
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ (1996) Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem 271:89–96 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases