Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds - PubMed (original) (raw)
doi: 10.1086/338625. Epub 2001 Dec 17.
Claire Fieschi, Frédéric Altare, Suliman Al-Jumaah, Sami Al-Hajjar, Jacqueline Feinberg, Stéphanie Dupuis, Claire Soudais, Ibrahim Zaid Al-Mohsen, Emmanuelle Génin, David Lammas, Dinakantha S Kumararatne, Tony Leclerc, Arash Rafii, Husn Frayha, Belinda Murugasu, Lee Bee Wah, Raja Sinniah, Michael Loubser, Emi Okamoto, Abdulaziz Al-Ghonaium, Haysam Tufenkeji, Laurent Abel, Jean-Laurent Casanova
Affiliations
- PMID: 11753820
- PMCID: PMC384913
- DOI: 10.1086/338625
Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds
Capucine Picard et al. Am J Hum Genet. 2002 Feb.
Abstract
Interleukin-12 (IL12) is a cytokine that is secreted by activated phagocytes and dendritic cells and that induces interferon-gamma production by natural-killer and T lymphocytes. It consists of two subunits, p35 and p40, which are encoded by IL12A and IL12B, respectively. The first reported patient with a genetic cytokine disorder was a Pakistani child, who was homozygous for a large loss-of-function deletion (g.482+82_856-854del) in IL12B. This IL12-deficient child suffered from infections caused by bacille Calmette-Guérin (BCG) and Salmonella enteritidis. We herein report 12 additional patients from five other kindreds. In one kindred from India, the same large deletion that was described elsewhere (g.482+82_856-854del) was identified. In four kindreds from Saudi Arabia, a recessive loss-of-function frameshift insertion (g.315_316insA) was found. A conserved haplotype encompassing the IL12B gene suggested that a founder effect accounted for the recurrence of each mutation. The two founder mutational events-g.482+82_856-854del and g.315_316insA-were estimated to have occurred approximately 700 and approximately 1,100 years ago, respectively. Among a total of 13 patients with IL12 deficiency, 1 child had salmonellosis only and 12 suffered from clinical disease due to BCG or environmental nontuberculous mycobacteria. One patient also had clinical disease caused by virulent Mycobacterium tuberculosis, five patients had clinical disease caused by Salmonella serotypes, and one patient had clinical disease caused by Nocardia asteroides. The clinical outcome varies from case to case, since five patients (aged 2-11 years) died of overwhelming infection, whereas eight patients (aged 3-12 years) are still in good health and are not currently taking antibiotics. In conclusion, IL12 deficiency is not limited to a single kindred, shows significant variability of outcome, and should be considered in the genetic diagnosis of patients with mycobacteriosis and/or salmonellosis. To date, two founder IL12B mutations have been identified, accounting for the recurrence of a large deletion and a small insertion within populations from the Indian subcontinent and from the Arabian Peninsula, respectively.
Figures
Figure 1
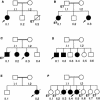
Pedigrees of six families with IL12 deficiency caused by IL12B mutations. Each kindred is designated by a capital letter (A–F), each generation is designated by a roman numeral (I or II), and each individual is designated by an arabic numeral (1–8). Patients with mycobacteriosis and/or salmonellosis are represented by black symbols. The probands are indicated by arrows. “E?” indicates individuals for whom genetic analysis was not possible (e.g., B.II.1, who died of disseminated BCG infection at age 5 years).
Figure 2
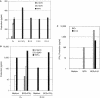
Impaired production of IL12. A, Production of IL12p70, IL12p40, and TNFα, by PDBu-stimulated EBV-B cells from a healthy positive control (C+) and a negative control (C−; A.II.1) (both of whom had previously been reported by Altare et al. [1998_c_]) and from patients (B.II.3, C.II.3, and D.II.4). Supernatants were harvested after 18 h of activation, for cytokine quantification by ELISA. The results are the mean ± SDs of two experiments. B, Production of IL12p70, IL12p40, and TNFα, by whole-blood cells from a healthy positive control (C+) and from patient D.II.4, either unstimulated (Medium) or stimulated with BCG plus IFNγ. Supernatants were harvested after 12 h of activation. C, Production of IFNγ by whole-blood cells from healthy positive control (C+) and from patient D.II.4, either unstimulated (Medium) or stimulated with BCG alone or with BCG plus recombinant IL12p70. The supernatants were harvested after 72 h of activation, for cytokine quantification by ELISA.
Figure 3
IL12B mutant alleles. A, Schematic representation of structural organization of coding region of wild-type (WT) IL12B gene. Exons I and VIII are not translated. Intrachain disulfite bounds and amino acids essential for dimerization with IL12p35 are indicated by the single-letter amino acid code. The positions of the two mutations found in IL12-deficient patients are also indicated. B, Schematic representation of coding region of two mutant IL12B alleles. Gray regions correspond to stretches of new amino acids resulting from the frameshift mutation.
Figure 4
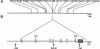
High-resolution map of IL12B locus. A, Physical map of IL12B region on chromosome 5q33. The physical distances (in Mb) between IL12B and microsatellite markers were obtained from BLAST. B, Genomic organization of human IL12B gene and location of intragenic SNPs. White boxes indicate coding exons; gray boxes indicate untranslated exons. Translation initiation and termination codons are indicated. Arrows indicate positions of intragenic SNPs (−1356, 364+92, and 1146).
Figure 5
Haplotypes encompassing IL12B in patients with IL12 deficiency. Markers encompassing IL12B, with physical distance (Mb) and recombination rate (θ), are shown. Physical distance was obtained from BLAST; the recombination rate was estimated by use of the correspondence of .672 cM for 1 Mb, over the whole region, and use of the Kosambi mapping function (see the “Dating of Mutations” subsection). The ancestral haplotypes shared by kindreds A and B, from the Indian subcontinent, is indicated by dark-gray shading; the ancestral haplotype shared by kindreds C–F, from Saudi Arabia, is indicated by intermediate-gray shading; and the ancestral haplotype shared by a subset of kindreds is indicated by light-gray shading.
Similar articles
- Inherited IL-12p40 deficiency: genetic, immunologic, and clinical features of 49 patients from 30 kindreds.
Prando C, Samarina A, Bustamante J, Boisson-Dupuis S, Cobat A, Picard C, AlSum Z, Al-Jumaah S, Al-Hajjar S, Frayha H, Al-Mousa H, Ben-Mustapha I, Adimi P, Feinberg J, de Suremain M, Jannière L, Filipe-Santos O, Mansouri N, Stephan JL, Nallusamy R, Kumararatne DS, Bloorsaz MR, Ben-Ali M, Elloumi-Zghal H, Chemli J, Bouguila J, Bejaoui M, Alaki E, AlFawaz TS, Al Idrissi E, ElGhazali G, Pollard AJ, Murugasu B, Wah Lee B, Halwani R, Al-Zahrani M, Al Shehri MA, Al-Zahrani M, Bin-Hussain I, Mahdaviani SA, Parvaneh N, Abel L, Mansouri D, Barbouche R, Al-Muhsen S, Casanova JL. Prando C, et al. Medicine (Baltimore). 2013 Mar;92(2):109-122. doi: 10.1097/MD.0b013e31828a01f9. Medicine (Baltimore). 2013. PMID: 23429356 Free PMC article. - Mendelian Susceptibility to Mycobacterial Disease Caused by a Novel Founder IL12B Mutation in Saudi Arabia.
Alodayani AN, Al-Otaibi AM, Deswarte C, Frayha HH, Bouaziz M, AlHelale M, Le Voyer T, Nieto-Patlan A, Rattina V, AlZahrani M, Halwani R, Al Sohime F, Al-Mousa H, Al-Muhsen S, Alhajjar SH, Dhayhi NS, Abel L, Casanova JL, Bin-Hussain I, AlBarrak MS, Al-Jumaah SA, Bustamante J. Alodayani AN, et al. J Clin Immunol. 2018 Apr;38(3):278-282. doi: 10.1007/s10875-018-0490-2. Epub 2018 Mar 27. J Clin Immunol. 2018. PMID: 29589181 Free PMC article. - A 1,100-year-old founder effect mutation in IL12B gene is responsible for Mendelian susceptibility to mycobacterial disease in Tunisian patients.
Ben-Mustapha I, Ben-Ali M, Mekki N, Patin E, Harmant C, Bouguila J, Elloumi-Zghal H, Harbi A, Béjaoui M, Boughammoura L, Chemli J, Barbouche MR. Ben-Mustapha I, et al. Immunogenetics. 2014 Jan;66(1):67-71. doi: 10.1007/s00251-013-0739-0. Epub 2013 Oct 15. Immunogenetics. 2014. PMID: 24127073 - Genetically determined susceptibility to mycobacterial infection.
Patel SY, Doffinger R, Barcenas-Morales G, Kumararatne DS. Patel SY, et al. J Clin Pathol. 2008 Sep;61(9):1006-12. doi: 10.1136/jcp.2007.051201. Epub 2008 Mar 6. J Clin Pathol. 2008. PMID: 18326015 Review. - Impaired interferon gamma-mediated immunity and susceptibility to mycobacterial infection in childhood.
Remus N, Reichenbach J, Picard C, Rietschel C, Wood P, Lammas D, Kumararatne DS, Casanova JL. Remus N, et al. Pediatr Res. 2001 Jul;50(1):8-13. doi: 10.1203/00006450-200107000-00005. Pediatr Res. 2001. PMID: 11420412 Review.
Cited by
- Bacille Calmette-Guérin vaccination in Saudi Arabia: benefits versus risks.
Al Jumaah S, Al Hajjar S, Al Mousa H. Al Jumaah S, et al. Ann Saudi Med. 2012 Jan-Feb;32(1):1-3. doi: 10.5144/0256-4947.2012.1. Ann Saudi Med. 2012. PMID: 22156632 Free PMC article. No abstract available. - TB vaccine development and the End TB Strategy: importance and current status.
Fletcher HA, Schrager L. Fletcher HA, et al. Trans R Soc Trop Med Hyg. 2016 Apr;110(4):212-8. doi: 10.1093/trstmh/trw016. Trans R Soc Trop Med Hyg. 2016. PMID: 27076508 Free PMC article. Review. - Inherited disorders of the IL-12-IFN-gamma axis in patients with disseminated BCG infection.
Mansouri D, Adimi P, Mirsaeidi M, Mansouri N, Khalilzadeh S, Masjedi MR, Adimi P, Tabarsi P, Naderi M, Filipe-Santos O, Vogt G, de Beaucoudrey L, Bustamante J, Chapgier A, Feinberg J, Velayati AA, Casanova JL. Mansouri D, et al. Eur J Pediatr. 2005 Dec;164(12):753-7. doi: 10.1007/s00431-005-1689-9. Epub 2005 Aug 10. Eur J Pediatr. 2005. PMID: 16091917 - Novel STAT1 alleles in otherwise healthy patients with mycobacterial disease.
Chapgier A, Boisson-Dupuis S, Jouanguy E, Vogt G, Feinberg J, Prochnicka-Chalufour A, Casrouge A, Yang K, Soudais C, Fieschi C, Santos OF, Bustamante J, Picard C, de Beaucoudrey L, Emile JF, Arkwright PD, Schreiber RD, Rolinck-Werninghaus C, Rösen-Wolff A, Magdorf K, Roesler J, Casanova JL. Chapgier A, et al. PLoS Genet. 2006 Aug 18;2(8):e131. doi: 10.1371/journal.pgen.0020131. PLoS Genet. 2006. PMID: 16934001 Free PMC article.
References
Electronic-Database Information
- BLAST, http://www.ncbi.nlm.nih.gov/BLAST/ (for localization of microsatellites markers from the chromosome 5 and IL12B sequences)
- Généthon Map, ftp://ftp.genethon.fr/pub/Gmap/Nature-1995/data/data_chrom5 (for microsatellites markers from the chromosome 5 region)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for familial atypical mycobacteriosis [MIM 209950])
References
- Aksu G, Trpan C, Çavuolu C, Soydan S, Altare F, Casanova JL, Kutukculer N (2001) Mycobacterium fortuitum-chelonae complex infection in a child with complete interleukin-12 receptor β1 deficiency. Pediatr Infect Dis J 20:551–553 - PubMed
- Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, Jeppsson O, Gollob JA, Meinl E, Segal AW, Fischer A, Kumararatne D, Casanova JL (1998a) Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432–1435 - PubMed
- Altare F, Ensser A, Breiman A, Reichenbach J, El Baghdadi J, Fischer A, Emile JF, Gaillard JL, Casanova JL (2001) IL-12 deficiency in a patient with abdominal tuberculosis. J Infect Dis 184:231–236 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials