Activation of Rac GTPase by p75 is necessary for c-jun N-terminal kinase-mediated apoptosis - PubMed (original) (raw)
Activation of Rac GTPase by p75 is necessary for c-jun N-terminal kinase-mediated apoptosis
Anthony W Harrington et al. J Neurosci. 2002.
Abstract
The neurotrophin receptor p75 can induce apoptosis both in vitro and in vivo. The mechanisms by which p75 induces apoptosis have remained mostly unknown. Here, we report that p75 activates Rac GTPase, which in turn activates c-jun N-terminal kinase (JNK), including an injury-specific JNK3, in an NGF-dependent manner. N17Rac blocks this JNK activation and subsequent NGF-dependent apoptosis, indicating that activation of Rac GTPase is required for JNK activation and apoptosis induced by p75. In addition, p75-mediated Rac activation is modulated by coactivation of Trk, identifying Rac GTPase as one of the key molecules whose activity is critical for cell survival and death in neurotrophin signaling. The crucial role of the JNK pathway in p75 signaling is further confirmed by the results that blocking p75 from signaling via the JNK pathway or suppressing the JNK activity itself led to inhibition of NGF-dependent death. Together, these results indicate that the apoptotic machinery of p75 comprises Rac GTPase and JNK.
Figures
Fig. 1.
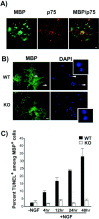
Oligodendrocytes from p75 null mice fail to die after NGF treatment. A, Mouse oligodendrocytes express p75 in culture, as do their rat counterparts. A representative picture shows a mouse oligodendrocyte culture taken from the wild-type mice at postnatal day 16 cortex. p75 expression on the cell surface was detected using 9651 anti-p75 antibody (red), and oligodendrocytes were identified by MBP stain (green). Scale bar, 8 μm. B, Oligodendrocytes fail to die in the absence of p75 when NGF is added. At 4–6 d after plating, mouse oligodendrocytes were treated with 100 ng/ml of NGF for 4 hr, fixed, and stained for MBP. Pyknotic cells among the MBP+ cells are indicated by_arrows_ and also shown at higher magnification in the_inset_. Scale bar, 20 μm. C, Quantification of TUNEL+ cells among MBP+ cells. The quantitation data are from two to four independent experiments, each with 100–150 cells counted for a total of 200–600 cells. WT, Wild-type;KO, knock-out.
Fig. 2.
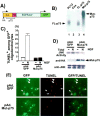
The signaling ability of p75 is required for NGF-dependent apoptosis in oligodendrocytes. A, Schematic diagram of the mutant-p75 lacking the cytoplasmic domain. The cytoplasmic domain of this mutant receptor was replaced with the corresponding domain of the kinase-dead EGF receptor. The_arrows_ indicate two independent CMV promoters, one directing expression of the mutant p75 and the other directing the expression of GFP. All the adenoviruses used in this study coexpress GFP. B, The mutant p75 binds 125I-NT3. Cos cells were infected with the full-length (FL) p75 or the mutant (Mut) p75 adenovirus and subjected to cross-linking with 125I-NT3. PC12 cells were used as a positive control (lane 1), and uninfected Cos cells were used as a negative control (lane 2). The FL-p75 was immunoprecipitated with 9992 antibody (lane 3), and the mutant p75 was immunoprecipitated with HA antibody (lane 4). C, The mutant p75 protects oligodendrocytes from NGF-dependent apoptosis. Oligodendrocytes were infected with GFP control or the mutant p75 adenovirus in four-well slide dishes for 24 hr at 150 pfu/cell. After 4 hr of NGF treatment, cells were stained for TUNEL. The number of TUNEL+cells was determined among GFP+ cells. The quantitation data are from three to five independent experiments, each with 200–300 cells counted for a total of 600–1500 cells.D, The mutant-p75 inhibits JNK activation in oligodendrocytes. Twenty-four hours after infection with the viruses, oligodendrocytes were treated with NGF at 100 ng/ml for 4 hr. The changes in JNK activity were measured by solid-phase kinase assays. The presence of the mutant-p75 was detected with anti-HA antibody, and the presence of the JNK protein was detected with anti-JNK antibody.E, Representative picture of oligodendrocytes quantified after infection with adenoviruses and NGF treatment. The cells expressing the mutant p75 were identified by GFP fluorescence, because the virus also expresses GFP as well as the mutant p75 cDNA. The_arrows_ indicate the TUNEL+ cells among GFP+ cells. Scale bars, 20 μm._EGFR-kin_−, A kinase-dead EGF receptor;Ext, extracellular domain.
Fig. 3.
p75 activates JNK1 and 3. A, Specificity of antibodies used in IP/K assays. 293 cells were transfected with HA-JNK1, HA-JNK2, or Flag-JNK3 cDNAs. The lysates from each transfected dish were subjected to immunoprecipitation reactions using anti-JNK1 (C17, polyclonal; Santa Cruz Biotechnologies), anti-JNK1 (G151, monoclonal; PharMingen), anti-JNK2 (Santa Cruz Biotechnologies), and anti-JNK3 (Upstate Biotechnology) antibodies. The immunoprecipitated proteins were detected using either anti-HA (JNK1 and 2) or anti-Flag (JNK3) antibody. B, Summary of the data presented in Figure 4. C, The lysates from rat oligodendrocytes were subjected to IP/K assays using the four antibodies. p75 activates JNK1, based on C17 and G151 antibodies, and JNK3, based on C17 and JNK3 antibodies.
Fig. 4.
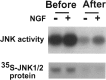
p75 activates JNK3 after depletion of JNK1 and 2. The oligodendrocyte lysates were subjected to immunodepletion to remove JNK1 and 2 using JNK1 and 2 antibodies. The depleted lysates were used in solid-phase kinase assays (top panel). The efficiency of immunodepletion is shown in the bottom panel. The extent of immunodepletion of JNK1 and 2 proteins was determined using 35S-JNK1 and JNK2 that were added together as a tracer to the oligodendrocyte lysates.
Fig. 5.
JNK activation is necessary for NGF-dependent apoptosis in oligodendrocytes. A, DN-JNK2 inhibits NGF-dependent activation of JNK in oligodendrocytes. Oligodendrocytes were infected with GFP control or DN-JNK2 adenovirus for 24 hr at 150 pfu/cell. Infected cells were untreated or treated with NGF for 4 hr, and the lysates were subjected to solid-phase kinase as well as IP/K assays. The presence of DN-JNK2 is shown in an HA Western blot, and the JNK protein is shown in a JNK Western blot. B, DN-JNK2 rescues oligodendrocytes from NGF-mediated apoptosis. The quantification procedure was identical to what was described in the legend of Figure 2.
Fig. 6.
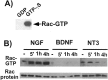
p75 activates Rac1 in an NGF-dependent manner.A, Specificity of the pull-down Rac assay. The lysates from 293 cells were incubated with either 1 m
m
GDP or 0.1 m
m
GTPγS and subjected to a pull-down assay using GST-PBD. The bound Rac protein was detected in a Western blot analysis with anti-Rac1 antibody. B, NGF addition led to a prolonged activation of Rac1 in oligodendrocytes. Oligodendrocytes were treated for the indicated time with NGF, BDNF, or NT3 at 100 ng/ml. The lysates were subjected to Rac activity assays. Note that BDNF does not activate Rac1, whereas NT3 activates it transiently.
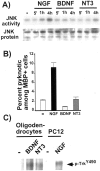
Fig. 7.
Trk activation intercepts p75-mediated JNK activity at or upstream of Rac GTPase. A, Temporal course of JNK activation by neurotrophins. Rat oligodendrocytes were treated with neurotrophins for the indicated time at 100 ng/ml. The lysates were subjected to solid-phase kinase assays. B, Only NGF is capable of inducing cell death among oligodendrocytes. Mouse oligodendrocytes were treated with 100 ng/ml NGF, BDNF, or NT3 for 4–5 hr, and the number of pyknotic cells was counted among MBP+ cells. The quantitation data are from three independent experiments, each with 100–200 cells counted for a total of 300–600 cells. C, Rat oligodendrocytes express TrkB and TrkC. Rat oligodendrocytes were untreated or treated with 100 ng/ml BDNF or NT3 for 5 min. The activated receptors were detected using phospho-TrkY490 antibody. Active TrkA from PC12 cells was used as a positive control (lanes 4, 5).
Fig. 8.
Rac1 is the upstream regulator of the p75-mediated JNK pathway. A, DN-Rac1 protects oligodendrocytes from NGF-dependent apoptosis. The procedure is identical to the one described in the legend of Figure 2. B, DN-Rac1 inhibits NGF-dependent JNK activation. Oligodendrocytes were infected with either GFP or DN-Rac1 for 24 hr and either left untreated or treated with 100 ng/ml NGF for 4 hr. The resulting lysates were used in solid-phase kinase assays.
Similar articles
- Direct inhibition of c-Jun N-terminal kinase in sympathetic neurones prevents c-jun promoter activation and NGF withdrawal-induced death.
Eilers A, Whitfield J, Shah B, Spadoni C, Desmond H, Ham J. Eilers A, et al. J Neurochem. 2001 Mar;76(5):1439-54. doi: 10.1046/j.1471-4159.2001.00150.x. J Neurochem. 2001. PMID: 11238729 - Neurotrophin receptor interacting factor (NRIF) is an essential mediator of apoptotic signaling by the p75 neurotrophin receptor.
Linggi MS, Burke TL, Williams BB, Harrington A, Kraemer R, Hempstead BL, Yoon SO, Carter BD. Linggi MS, et al. J Biol Chem. 2005 Apr 8;280(14):13801-8. doi: 10.1074/jbc.M410435200. Epub 2005 Jan 24. J Biol Chem. 2005. PMID: 15668238 - Inhibition of Rac GTPase triggers a c-Jun- and Bim-dependent mitochondrial apoptotic cascade in cerebellar granule neurons.
Le SS, Loucks FA, Udo H, Richardson-Burns S, Phelps RA, Bouchard RJ, Barth H, Aktories K, Tyler KL, Kandel ER, Heidenreich KA, Linseman DA. Le SS, et al. J Neurochem. 2005 Aug;94(4):1025-39. doi: 10.1111/j.1471-4159.2005.03252.x. J Neurochem. 2005. PMID: 16092944 Free PMC article. - The p75 neurotrophin receptor and neuronal apoptosis.
Barrett GL. Barrett GL. Prog Neurobiol. 2000 Jun;61(2):205-29. doi: 10.1016/s0301-0082(99)00056-8. Prog Neurobiol. 2000. PMID: 10704998 Review. - Role of JNK in tumor development.
Kennedy NJ, Davis RJ. Kennedy NJ, et al. Cell Cycle. 2003 May-Jun;2(3):199-201. Cell Cycle. 2003. PMID: 12734425 Review.
Cited by
- 3,4-methylenedioxyamphetamine upregulates p75 neurotrophin receptor protein expression in the rat brain.
Wang C, Peng Z, Kuang W, Zheng H, Long J, Wang X. Wang C, et al. Neural Regen Res. 2012 Apr 25;7(12):955-9. doi: 10.3969/j.issn.1673-5374.2012.12.013. Neural Regen Res. 2012. PMID: 25722682 Free PMC article. - Suppression of the p75 neurotrophin receptor in uninjured sensory neurons reduces neuropathic pain after nerve injury.
Obata K, Katsura H, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Noguchi K. Obata K, et al. J Neurosci. 2006 Nov 15;26(46):11974-86. doi: 10.1523/JNEUROSCI.3188-06.2006. J Neurosci. 2006. PMID: 17108171 Free PMC article. - Therapeutic Potential of Neurotrophins for Repair After Brain Injury: A Helping Hand From Biomaterials.
Houlton J, Abumaria N, Hinkley SFR, Clarkson AN. Houlton J, et al. Front Neurosci. 2019 Aug 2;13:790. doi: 10.3389/fnins.2019.00790. eCollection 2019. Front Neurosci. 2019. PMID: 31427916 Free PMC article. Review. - Aβ selectively impairs mGluR7 modulation of NMDA signaling in basal forebrain cholinergic neurons: implication in Alzheimer's disease.
Gu Z, Cheng J, Zhong P, Qin L, Liu W, Yan Z. Gu Z, et al. J Neurosci. 2014 Oct 8;34(41):13614-28. doi: 10.1523/JNEUROSCI.1204-14.2014. J Neurosci. 2014. PMID: 25297090 Free PMC article. - Nerve Growth Factor (NGF) modulates in vitro induced myofibroblasts by highlighting a differential protein signature.
Esposito G, Balzamino BO, Stigliano E, Biamonte F, Urbani A, Micera A. Esposito G, et al. Sci Rep. 2021 Jan 18;11(1):1672. doi: 10.1038/s41598-021-81040-x. Sci Rep. 2021. PMID: 33462282 Free PMC article.
References
- Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. - PubMed
- Brennan C, Rivas-Plata K, Landis SC. The p75 neurotrophin receptor influences NT-3 responsiveness of sympathetic neurons in vivo. Nat Neurosci. 1999;2:699–705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous