Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior - PubMed (original) (raw)
Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior
Tracy L Bale et al. J Neurosci. 2002.
Abstract
Corticotropin-releasing factor (CRF) and its family of peptides are critical coordinators of homeostasis whose actions are mediated through their receptors, CRF receptor 1 (CRFR1) and CRFR2, found throughout the CNS and periphery. The phenotypes of mice deficient in either CRFR1 or CRFR2 demonstrate the critical role these receptors play. CRFR1-mutant mice have an impaired stress response and display decreased anxiety-like behavior, whereas CRFR2-mutant mice are hypersensitive to stress and display increased anxiety-like behavior. To further elucidate the roles of both CRF receptors and determine their interaction in behaviors, we have generated mice deficient in both CRFR1 and CRFR2. The behavioral phenotype of these mice demonstrates a novel role of the mother's genotype on development of pup anxiety. We have found that although the female double-mutant mice display anxiolytic-like behavior, the male double-mutant mice show significantly more anxiety-like behavior compared with the females. We have also determined that the dam's CRFR2 genotype affects the anxiety-like behavior of the male mice, such that a pup born to a heterozygous or mutant dam displays significantly more anxiety-like behavior regardless of that pup's genotype. Double-mutant mice also display an even greater impairment of their hypothalamic-pituitary-adrenal axis response to stress than that of the CRFR1-mutant mice. CRF mRNA levels are elevated in CRFR1- and double-mutant mice, and urocortin III and vasopressin mRNA levels are increased in CRFR2- and double-mutant mice. These results indicate that both CRFR1 and CRFR2 have critical roles in gene regulation and the maintenance of homeostasis in response to stress.
Figures
Fig. 1.
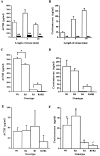
Impairment of the HPA axis response to stress in double-mutant animals. Time course of restraint stress effects on ACTH (A) and corticosterone (B) plasma levels at 9:00 A.M. (n = 8 for wild-type mice, white bars; n = 7 for double-mutant mice, black bars; mean ± SEM). **Significantly different from wild-type controls at the same time point; p < 0.001 by ANOVA, Scheffe's post hoc test. Comparison of ACTH (C) and corticosterone (D) plasma levels in control, CRFR1-, CRFR2-, and CRFR1/2-mutant mice after 10 min of restraint stress (n = 4; mean ± SEM; *p < 0.05 by ANOVA, Fisher's post hoc test). Basal ACTH (E) and corticosterone (F) plasma levels (taken by retro-orbital eyebleed at 08:00 A.M.) for wild-type, CRFR1-, CRFR2-, and CRFR1/2-mutant male mice. No statistical differences were found between groups for ACTH levels. For corticosterone, _a_was significantly different from b and c(p < 0.01) and b was significantly different from c(p < 0.05 by ANOVA, Scheffe's post hoc test) (n = 4 for wild-type and CRFR2 mice; n = 3 for CRFR1 and CRFR1/2 mice; mean ± SEM).
Fig. 2.
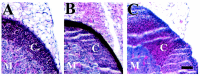
Double-mutant adrenal glands have atrophied cortex, similar to CRFR1 adrenal glands. H&E staining of the adrenal gland from double-mutant (A), CRFR1-mutant (B), and wild-type (C) mice is shown. Note that there is no difference in adrenal gland cortex size between the CRFR1-mutant mice and the double-mutant mice.C, Cortex; M, medulla.n = 3. Scale bar, 50 μm.
Fig. 3.
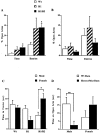
Sexual dichotomy in anxiety-like behaviors of double-mutant mice. Decreased anxiety-like behavior of double-mutant animals as measured in the elevated plus maze (A, B) test of anxiety is shown. All tests were conducted on individually housed 16- to 20-week-old female and male mice. All results are reported as mean ± SEM. A, Percentage of time spent in the open arms and number of entries into the open arms were significantly more for the female double-mutant mice (n = 27) than for the wild-type controls (n = 27) (*p < 0.05). Female CRFR1-mutant mice were not significantly different from controls (n = 4). B, Percentage of time spent in the open arms and number of entries into the open arms were not significantly different between male double-mutant mice (n = 11), CRFR1-mutant mice (_n_= 8), and wild-type mice (n = 10).C, Comparison of males and females on the open-field test. Male double-mutant mice spend significantly less time in the center of the open field than double-mutant females (n = 10 males; n = 16 females; *p < 0.05). No significant differences were found between male and female wild-type mice (n = 10 males; n = 24 females; p = 0.63) or CRFR1-mutant mice (n = 8 males;n = 5 for females; p = 0.06).D, A significant correlation was found between the dam's CRFR2 genotype and the male pup's anxiety-like behavior. If the mother was heterozygous or homozygous for CRFR2 mutation, the pup, regardless of its genotype, showed significantly more anxiety-like behavior (as measured by time spent on the open arm of the elevated plus maze). **Significantly different (p < 0.05). For male pups born to wild-type dams, n = 4 CRFR1-mutant mice and n = 6 wild-type mice. For male pups born to either heterozygous or mutant dams,n = 4 CRFR1-mutant mice, n = 11 double-mutant mice, and n = 4 wild-type mice. For female pups born to wild-type dams, n = 3 CRFR1-mutant mice and n = 18 wild-type mice. For female pups born to either heterozygous or mutant dams,n = 2 CRFR1-mutant mice, n = 27 double-mutant mice, and n = 9 wild-type mice.
Fig. 4.
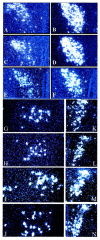
CRF, Ucn III, and VP mRNA levels are increased in the absence of both receptors Silver grains by _in situ_hybridization for CRF mRNA in the PVN (A–F), Ucn III mRNA in the lateral perifornical region (G–J), and VP in the PVN (K–N) comparing wild-type mice (A, E, G, K), CRFR1-mutant mice (B, H, L), CRFR2-mutant mice (C, I, M), and double-mutant mice (D, F, J, N) are shown. Double-mutant mice were also treated with corticosterone (F) and compared with wild-type mouse CRF mRNA levels (E). Corticosterone-treated mice were given 25 μg/ml corticosterone in their drinking water for 14 d before they were killed.
Similar articles
- Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development.
Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Smith GW, et al. Neuron. 1998 Jun;20(6):1093-102. doi: 10.1016/s0896-6273(00)80491-2. Neuron. 1998. PMID: 9655498 - Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress.
Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Bale TL, et al. Nat Genet. 2000 Apr;24(4):410-4. doi: 10.1038/74263. Nat Genet. 2000. PMID: 10742108 - The role of corticotropin-releasing factor receptors in stress and anxiety.
Bale TL, Lee KF, Vale WW. Bale TL, et al. Integr Comp Biol. 2002 Jul;42(3):552-5. doi: 10.1093/icb/42.3.552. Integr Comp Biol. 2002. PMID: 21708750 - CRF and CRF receptors: role in stress responsivity and other behaviors.
Bale TL, Vale WW. Bale TL, et al. Annu Rev Pharmacol Toxicol. 2004;44:525-57. doi: 10.1146/annurev.pharmtox.44.101802.121410. Annu Rev Pharmacol Toxicol. 2004. PMID: 14744257 Review. - The CRF Family of Neuropeptides and their Receptors - Mediators of the Central Stress Response.
Dedic N, Chen A, Deussing JM. Dedic N, et al. Curr Mol Pharmacol. 2018;11(1):4-31. doi: 10.2174/1874467210666170302104053. Curr Mol Pharmacol. 2018. PMID: 28260504 Free PMC article. Review.
Cited by
- Neuroanatomical evidence and a mouse calcitonin gene-related peptide model in line with human functional magnetic resonance imaging data support the involvement of peptidergic Edinger-Westphal nucleus in migraine.
Al-Omari A, Gaszner B, Zelena D, Gecse K, Berta G, Biró-Sütő T, Szocsics P, Maglóczky Z, Gombás P, Pintér E, Juhász G, Kormos V. Al-Omari A, et al. Pain. 2024 Jun 14;165(12):2774-93. doi: 10.1097/j.pain.0000000000003294. Online ahead of print. Pain. 2024. PMID: 38875125 Free PMC article. - CRF regulates pain sensation by enhancement of corticoaccumbal excitatory synaptic transmission.
Zhao W, Yu YM, Wang XY, Xia SH, Ma Y, Tang H, Tao M, Li H, Xu Z, Yang JX, Wu P, Zhang H, Ding HL, Cao JL. Zhao W, et al. Mol Psychiatry. 2024 Jul;29(7):2170-2184. doi: 10.1038/s41380-024-02488-7. Epub 2024 Mar 7. Mol Psychiatry. 2024. PMID: 38454083 - Sex Differences in Stress Response: Classical Mechanisms and Beyond.
Hodes GE, Bangasser D, Sotiropoulos I, Kokras N, Dalla C. Hodes GE, et al. Curr Neuropharmacol. 2024;22(3):475-494. doi: 10.2174/1570159X22666231005090134. Curr Neuropharmacol. 2024. PMID: 37855285 Free PMC article. - To Predict, Prevent, and Manage Post-Traumatic Stress Disorder (PTSD): A Review of Pathophysiology, Treatment, and Biomarkers.
Al Jowf GI, Ahmed ZT, Reijnders RA, de Nijs L, Eijssen LMT. Al Jowf GI, et al. Int J Mol Sci. 2023 Mar 9;24(6):5238. doi: 10.3390/ijms24065238. Int J Mol Sci. 2023. PMID: 36982313 Free PMC article. Review. - Mother-Young Bonding: Neurobiological Aspects and Maternal Biochemical Signaling in Altricial Domesticated Mammals.
Bienboire-Frosini C, Marcet-Rius M, Orihuela A, Domínguez-Oliva A, Mora-Medina P, Olmos-Hernández A, Casas-Alvarado A, Mota-Rojas D. Bienboire-Frosini C, et al. Animals (Basel). 2023 Feb 2;13(3):532. doi: 10.3390/ani13030532. Animals (Basel). 2023. PMID: 36766424 Free PMC article. Review.
References
- Allen JP, Allen CF. Amygdalar participation in tonic ACTH secretion in the rat. Neuroendocrinology. 1975;19:115–125. - PubMed
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. - PubMed
- Beaulieu S, Di Paolo T, Barden N. Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology. 1986;44:247–254. - PubMed
- Bilezikjian LM, Vale WW. Regulation of ACTH secretion from corticotrophs: the interaction of vasopressin and CRF. Ann NY Acad Sci. 1987;512:85–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases