Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis - PubMed (original) (raw)
Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis
Anthony R West et al. J Neurosci. 2002.
Abstract
The tonic influence of dopamine D1 and D2 receptors on the activity of striatal neurons in vivo was investigated by performing intracellular recordings concurrently with reverse microdialysis in chloral hydrate-anesthetized rats. Striatal neurons were recorded in the vicinity of the microdialysis probe to assess their activity during infusions of artificial CSF (aCSF), the D1 receptor antagonist SCH 23390 (10 microm), or the D2 receptor antagonist eticlopride (20 microm). SCH 23390 perfusion decreased the excitability of striatal neurons exhibiting electrophysiological characteristics of spiny projection cells as evidenced by a decrease in the maximal depolarized membrane potential, a decrease in the amplitude of up-state events, and an increase in the intracellular current injection amplitude required to elicit an action potential. Conversely, a marked depolarization of up- and down-state membrane potential modes, a decrease in the amplitude of intracellular current injection required to elicit an action potential, and an increase in the number of spikes evoked by depolarizing current steps were observed in striatal neurons after local eticlopride infusion. A significant increase in maximal EPSP amplitude evoked by electrical stimulation of the prefrontal cortex was also observed during local eticlopride but not SCH 23390 infusion. These results indicate that in intact systems, ongoing dopaminergic neurotransmission exerts a powerful tonic modulatory influence on the up- and down-state membrane properties of striatal neurons and controls their excitability differentially via both D1- and D2-like receptors. Moreover, a significant component of D2 receptor-mediated inhibition of striatal neuron activity in vivo occurs via suppression of excitatory synaptic transmission.
Figures
Fig. 1.
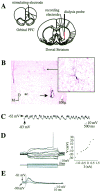
Intracellular recordings from striatal neurons located proximal to the microdialysis probe. A, Positioning of implants. Electrodes and microdialysis probes were stereotaxically implanted using a micromanipulator (see Materials and Methods). All coordinates were derived from the stereotaxic atlas ofPaxinos and Watson (1986). The corticostriatal pathway from the orbital PFC to the central striatum was activated in some experiments via electrical stimulation. B, Left, Coronal section (2.5×) depicting a photomontage of a striatal neuron (solid arrow) labeled after intracellular biocytin injection (enlarged to 20× on the right). Note that the neuron was located proximal to the active zone of the microdialysis probe (extends dorsally 4 mm from the termination point of the probe track indicated by the dashed arrow). ac, Anterior commissure. C, Intracellular recordings from the striatal neuron labeled in B revealed that this cell did not fire spontaneously but did exhibit membrane activity characterized by rapid and spontaneous transitions from a hyperpolarized state to a depolarized plateau. D,Left, In the same cell, intracellular injection of 150 msec duration constant current pulses (bottom traces) induced deflections in the membrane potential (top traces). Right, A plot of the steady-state voltage deflections against the current pulse amplitudes derived from recordings shown on the left. The input resistance of this neuron (as well as the mean input resistance of all neurons recorded proximal to the dialysis probe) was very similar to that observed in intact animals. E, Single pulses of electrical stimulation delivered to the PFC evoked short-latency EPSPs in this same striatal neuron in a stimulus amplitude-dependent manner.
Fig. 2.
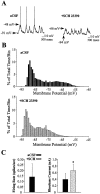
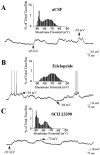
Intrastriatal SCH 23390 infusion attenuates the excitability of striatal neurons. A,Left, During aCSF (vehicle) infusion, this striatal neuron exhibits rapid spontaneous shifts in steady-state membrane potential and spontaneous spike discharge. Right, During local SCH 23390 infusion (10 μ
m
, 10 min), this same cell exhibits a hyperpolarization of the membrane and cessation of action potential discharge. Arrows indicate the membrane potential at its maximal depolarized and hyperpolarized levels.B, Comparisons of time histograms of the membrane potential (1 mV bins) constructed from the same neuron recorded during separate periods of aCSF (top, black bars) and SCH 23390 (bottom, gray bars) infusion revealed a decrease in the maximal depolarized membrane potential and an overall hyperpolarizing shift in the time spent at a given membrane potential. C, The mean ± SEM firing rate and rheobase current were determined in striatal neurons recorded during intrastriatal aCSF and again after SCH 23390 (n = 6) infusion (5–20 min). Left, A cessation of action potential discharge was observed after local SCH infusion. Right, The average minimal current amplitude required to reach threshold (rheobase) was significantly increased after intrastriatal SCH 23390 infusion (*p < 0.005; paired _t_ test). SCH 23390 infusion did not significantly affect the membrane potential recorded before current injection (aCSF control = −70.4 mV; SCH 23390 = −79.8 mV;_p_ > 0.05, paired t test).
Fig. 3.
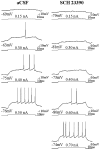
Intrastriatal SCH 23390 infusion decreases the responsiveness of single striatal neurons to intracellular current injection. Left column, Response of a single cell to increasing amplitudes of intracellular current injected during aCSF infusion. Right column, Response of the same cell to depolarizing current pulses injected during SCH 23390 infusion (∼6–8 min). Note that after SCH 23390 (10 μ
m
) infusion, the responsiveness of this cell to intracellular current injection was decreased even when the membrane potential before the current pulse was more depolarized than control conditions (third trace, 0.4 nA). Bottom traces indicate current injection steps.Top traces indicate the voltage response. The membrane potential before current injection is indicated below the voltage trace. The current amplitude is indicated above the current step.
Fig. 4.
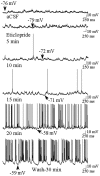
Intrastriatal eticlopride infusion increases the excitability of striatal neurons. A,Left, During aCSF (vehicle) infusion, this striatal neuron exhibits rapid spontaneous shifts in steady-state membrane potential but does not exhibit spontaneous spike discharge.Right, During local eticlopride infusion (20 μm, 4.5–5.5 min), the membrane potential of this same cell is depolarized, and the cell fires action potentials. Arrows indicate the membrane potential at its most depolarized and hyperpolarized levels. B, Comparisons of time histograms of the membrane potential constructed from the same neuron recorded during separate periods of aCSF (black bars) and eticlopride (white bars) infusion reveal a decrease in the maximal hyperpolarized membrane potential (1 mV bins), demonstrated by a rightward shift in the time spent at a given membrane potential.C, An increase in the mean ± SEM firing rate was observed in some neurons (2 of 5) recorded during local eticlopride infusion.
Fig. 5.
Time course of the excitatory effects of eticlopride on neuronal activity of a single striatal cell. During aCSF infusion the neuron is primarily hyperpolarized and is not firing action potentials (top trace). Approximately 5–10 min after eticlopride (20 μ
m
) infusion, the neuron depolarizes and begins to fire action potentials. The neuron is robustly activated after 20 min of eticlopride infusion and remains activated 30 min after the discontinuation of eticlopride infusion (aCSF wash, bottom trace).
Fig. 6.
Effects of local D1 and D2antagonist infusion on spontaneous activity recorded across cells. Striatal neurons were recorded after the initiation (10–90 min) of intrastriatal aCSF, eticlopride, or SCH 23390 infusion.A, During aCSF (vehicle) infusion, this striatal neuron exhibits rapid spontaneous shifts in steady-state membrane potential but does not exhibit spontaneous spike discharge. B, During local eticlopride infusion (20 μm, 10–90 min), the majority of neurons exhibited up- and down-state activity, and 40% of the cells fired action potentials. C, During intrastriatal SCH 23390 infusion (10 μm, 10–90 min), the majority of neurons exhibited up- and down-state activity; however, a significant leftward shift in the maximal depolarized membrane potential was observed (see_inset_). Insets show representative time histograms of the membrane potential of the same cells plotted over a 30 sec baseline period. The majority of neurons from all groups exhibited bimodal distributions in membrane potential characteristic of striatal projection cells. Arrows indicate the membrane potential at its maximal depolarized and hyperpolarized levels.
Fig. 7.
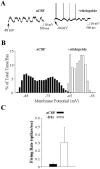
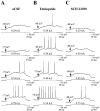
Intrastriatal dopamine antagonist infusion alters the responsiveness of single striatal neurons to intracellular current injection. A, Typical response of a single cell to gradually increasing amplitudes of intracellular current injected during aCSF infusion. B, Typical response of a cell to similar depolarizing current pulses injected during eticlopride (20 μ
m
) infusion (∼10–90 min). Note that after eticlopride infusion, the responsiveness of this cell to intracellular current injection was increased relative to across-cell aCSF controls. C, Typical response of a cell to similar depolarizing current pulses injected during SCH 23390 (10 μ
m
) infusion (∼10–90 min). Note that after SCH 23390 infusion, the responsiveness of this cell to intracellular current injection was decreased relative to across-cell aCSF controls.Bottom traces indicate current injection steps.Top traces indicate the voltage response. The membrane potential before current injection is indicated above the voltage trace. The current amplitude is indicated above the current step.
Fig. 8.
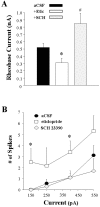
Opposite effects of local D1 and D2 antagonist infusion on activity evoked by intracellular injection of depolarizing current. The mean ± SEM rheobase current and number of spikes evoked by current steps of increasing intensity were determined in separate populations of striatal neurons recorded during intrastriatal aCSF, eticlopride (20 μ
m
), or SCH 23390 (10 μ
m
) infusion (5–90 min). Comparisons of the above measures of neuronal activity were made among the aCSF control (n = 19 cells), eticlopride (n = 10 cells), and SCH 23390 (n = 10) groups using a one-way ANOVA.A, Eticlopride infusion induced a decrease in the minimal current amplitude required to reach threshold (*p < 0.05; ANOVA, Dunn's test), whereas SCH 23390 was observed to increase the rheobase current (#_p_ < 0.05; ANOVA, Dunn's test)._B_, An overall increase in the number of spikes elicited for a given current intensity was observed after eticlopride infusion (*_p_ < 0.05; ANOVA, Dunnett's test), whereas SCH 23390 was without effect (_p_ > 0.05).
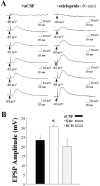
Fig. 9.
Intrastriatal eticlopride infusion increases the responsiveness of striatal neurons to electrical stimulation of the prefrontal cortex. A series of single pulses (0.2 Hz) of electrical stimuli were delivered at increasing stimulus intensities (0.2–3.0 mA) to striatal neurons recorded during intrastriatal aCSF, eticlopride (20 μ
m
), or SCH 23390 (10 μ
m
) infusion. A, Representative traces of EPSPs evoked by an increasing series of stimulus intensities in separate single striatal neurons during aCSF (left) and eticlopride (right) infusion. B, Comparisons of mean maximal EPSP amplitudes in control (23.3 ± 2.5 mV), eticlopride (30.6 ± 1.1 mV), and SCH 23390 (19.9 ± 4.3) groups revealed that the response evoked in striatal neurons after electrical stimulation of the PFC is increased during intrastriatal eticlopride (*p < 0.05; ANOVA, Dunnett's test) but not SCH 23390 (_p_ > 0.05) infusion.
Similar articles
- Striatal neuronal activity and responsiveness to dopamine and glutamate after selective blockade of D1 and D2 dopamine receptors in freely moving rats.
Kiyatkin EA, Rebec GV. Kiyatkin EA, et al. J Neurosci. 1999 May 1;19(9):3594-609. doi: 10.1523/JNEUROSCI.19-09-03594.1999. J Neurosci. 1999. PMID: 10212318 Free PMC article. - Membrane properties of striatal direct and indirect pathway neurons in mouse and rat slices and their modulation by dopamine.
Planert H, Berger TK, Silberberg G. Planert H, et al. PLoS One. 2013;8(3):e57054. doi: 10.1371/journal.pone.0057054. Epub 2013 Mar 1. PLoS One. 2013. PMID: 23469183 Free PMC article. - Functional pathology of neuroleptic-induced dystonia based on the striatal striosome-matrix dopamine system in humans.
Goto S. Goto S. J Neurol Neurosurg Psychiatry. 2025 Jan 16;96(2):177-183. doi: 10.1136/jnnp-2024-334545. J Neurol Neurosurg Psychiatry. 2025. PMID: 39631787 Review. - Brain oscillations, medium spiny neurons, and dopamine.
Murer MG, Tseng KY, Kasanetz F, Belluscio M, Riquelme LA. Murer MG, et al. Cell Mol Neurobiol. 2002 Dec;22(5-6):611-32. doi: 10.1023/a:1021840504342. Cell Mol Neurobiol. 2002. PMID: 12585682 Free PMC article. Review.
Cited by
- Inhibition of striatal soluble guanylyl cyclase-cGMP signaling reverses basal ganglia dysfunction and akinesia in experimental parkinsonism.
Tseng KY, Caballero A, Dec A, Cass DK, Simak N, Sunu E, Park MJ, Blume SR, Sammut S, Park DJ, West AR. Tseng KY, et al. PLoS One. 2011;6(11):e27187. doi: 10.1371/journal.pone.0027187. Epub 2011 Nov 2. PLoS One. 2011. PMID: 22073284 Free PMC article. - D2 dopamine modulation of corticoaccumbens synaptic responses changes during adolescence.
Benoit-Marand M, O'Donnell P. Benoit-Marand M, et al. Eur J Neurosci. 2008 Mar;27(6):1364-72. doi: 10.1111/j.1460-9568.2008.06107.x. Epub 2008 Mar 7. Eur J Neurosci. 2008. PMID: 18331340 Free PMC article. - Dopamine attenuates evoked inhibitory synaptic currents in central amygdala neurons.
Naylor JC, Li Q, Kang-Park MH, Wilson WA, Kuhn C, Moore SD. Naylor JC, et al. Eur J Neurosci. 2010 Dec;32(11):1836-42. doi: 10.1111/j.1460-9568.2010.07457.x. Epub 2010 Oct 19. Eur J Neurosci. 2010. PMID: 20955472 Free PMC article. - Dissociable effects of dopamine on neuronal firing rate and synchrony in the dorsal striatum.
Burkhardt JM, Jin X, Costa RM. Burkhardt JM, et al. Front Integr Neurosci. 2009 Oct 30;3:28. doi: 10.3389/neuro.07.028.2009. eCollection 2009. Front Integr Neurosci. 2009. PMID: 19949467 Free PMC article. - Roles of D1-like dopamine receptors in the nucleus accumbens and dorsolateral striatum in conditioned avoidance responses.
Wietzikoski EC, Boschen SL, Miyoshi E, Bortolanza M, Dos Santos LM, Frank M, Brandão ML, Winn P, Da Cunha C. Wietzikoski EC, et al. Psychopharmacology (Berl). 2012 Jan;219(1):159-69. doi: 10.1007/s00213-011-2384-3. Epub 2011 Jul 1. Psychopharmacology (Berl). 2012. PMID: 21720753
References
- Benveniste H, Hüttemeier PC. Microdialysis—theory and application. Prog Neurobiol. 1990;35:195–215. - PubMed
- Calabresi P, Mercuri NB, Stanzione P, Stefani A, Bernardi G. Intracellular studies on the dopamine-induced firing inhibition of neostriatal neurons in vitro: evidence for D1 receptor involvement. Neuroscience. 1987;20:757–771. - PubMed
- Calabresi P, Mercuri NB, Stefani A, Bernardi G. Synaptic and intrinsic control of membrane excitability of neostriatal neurons I. An in vivo analysis. J Neurophysiol. 1990;63:651–652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 NS010725/NS/NINDS NIH HHS/United States
- MH 57440/MH/NIMH NIH HHS/United States
- P50 MH045156/MH/NIMH NIH HHS/United States
- MH 45156/MH/NIMH NIH HHS/United States
- NS 10725/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous