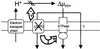A significant portion of mitochondrial proton leak in intact thymocytes depends on expression of UCP2 - PubMed (original) (raw)
A significant portion of mitochondrial proton leak in intact thymocytes depends on expression of UCP2
Stefan Krauss et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The uncoupling protein homologue UCP2 is expressed in a variety of mammalian cells. It is thought to be an uncoupler of oxidative phosphorylation. Uncoupling proteins previously have been shown to be capable of translocating protons across phospholipid bilayers in proteoliposome systems. Furthermore, studies in mitochondria from yeast overexpressing the proteins have led to suggestions that they may act as uncouplers in cells. However, this issue is controversial, and to date, definitive experimental evidence is lacking as to whether UCP2 mediates part or all of the basal mitochondrial proton leak in mammalian cells in situ. In the present study, by using thymocytes isolated from UCP2-deficient and wild-type (WT) mice, we addressed the question whether UCP2 is directly involved in catalyzing proton leak in intact cells. Over a range of mitochondrial membrane potentials (DeltaPsi(m)), proton leak activity was lower in thymocytes from UCP2-deficient mice compared with WT mice. At physiological levels of DeltaPsi(m), a significant portion (50%) of basal proton leak in resting cells depended on UCP2. Of note, proton leak in whole cells from WT mice, but not UCP2-deficient mice, responded to stimulation by 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-napthalenyl)-1-propenyl]benzoic acid (TTNPB), a known activator of UCP2 activity. Consistent with the observed changes in proton leak, DeltaPsi(m) and ATP levels were increased in untreated thymocytes from UCP2-deficient mice. Interestingly, resting respiration was unaltered, suggesting that UCP2 function in resting cells may be concerned with the control of ATP production rather than substrate oxidation. This study establishes that UCP2, expressed at endogenous levels, mediates proton leak in intact cells.
Figures
Figure 1
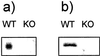
(a) RNA was extracted from thymocytes, and Northern blot analysis was performed with a full-length rat Ucp2 cDNA hybridization probe. (b) Mitochondria were isolated from thymus and immunoblotted for UCP2 protein.
Figure 2
FACS analysis of thymocytes from (a) WT and (b) UCP2-deficient mice. (c) Quantitative assessment of CD4/CD8 distributions shown in a and_b_.
Figure 3
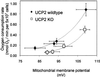
Proton leak kinetics in thymocytes from WT and UCP2-deficient mice. Titrations were performed as described in Materials and Methods. State 4 respiration (i.e., the cells do not synthesize ATP) was induced by incubation with 160 ng/ml oligomycin (top right-hand points of each curve). ΔΨm was altered by titrations with different concentrations of myxothiazol (40, 80, and 480 nM). Data are means ± SEM (n = 6 and_n_ = 5 cell preparations for WT and UCP2-deficient thymocytes, respectively). At steady state, the number of protons pumped by the complexes of the electron-transport chain equals the number of protons that leak back into the mitochondrial matrix. Because proton pumping and oxygen consumption are stoichiometrically linked, the oxygen consumption rates at any given potential reflect proton leak across the inner mitochondrial membrane.
Figure 4
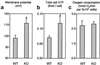
Comparison of ΔΨm, total cell ATP content, and oxygen consumption in resting thymocytes. ATP data are means ± SEM for 9- and 13-cell preparations from WT and UCP2-deficient mice, respectively. Respiration and ΔΨm data are means ± SEM for 7- and 5-cell preparations from WT and UCP2-deficient mice, respectively. *, P < 0.05.
Figure 5
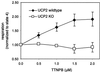
Effect of TTNPB on state 4 (i.e., nonphosphorylating) respiration in intact thymocytes. Thymocytes (at ≈7 × 107 cells per ml) were incubated with 160 ng/ml oligomycin, and respiration was measured. TTNPB was added in 0.5 μM increments, and respiration was measured after steady state was established.
Figure 6
Model of UCP2 function in resting thymocytes (see_Discussion_ for details). Protons are pumped into the mitochondrial intermembrane space by complexes of the electron transport chain during oxidation of substrates. UCP2 mediates a significant portion of mitochondrial proton leak in resting thymocytes, thus providing one of three possible routes for protons to re-enter the matrix (the other two being ATP synthase and UCP2-independent leak). UCP2 deficiency causes an increase in ΔΨm. This increase may either put backpressure on the proton pumps in the electron-transport chain (1), thus inhibiting substrate oxidation and oxygen consumption rates, or increase ATP production (2).
Similar articles
- Increased mitochondrial proton leak in skeletal muscle mitochondria of UCP1-deficient mice.
Monemdjou S, Hofmann WE, Kozak LP, Harper ME. Monemdjou S, et al. Am J Physiol Endocrinol Metab. 2000 Oct;279(4):E941-6. doi: 10.1152/ajpendo.2000.279.4.E941. Am J Physiol Endocrinol Metab. 2000. PMID: 11001779 - No evidence for a basal, retinoic, or superoxide-induced uncoupling activity of the uncoupling protein 2 present in spleen or lung mitochondria.
Couplan E, del Mar Gonzalez-Barroso M, Alves-Guerra MC, Ricquier D, Goubern M, Bouillaud F. Couplan E, et al. J Biol Chem. 2002 Jul 19;277(29):26268-75. doi: 10.1074/jbc.M202535200. Epub 2002 May 14. J Biol Chem. 2002. PMID: 12011051 - Retinoids activate proton transport by the uncoupling proteins UCP1 and UCP2.
Rial E, González-Barroso M, Fleury C, Iturrizaga S, Sanchis D, Jiménez-Jiménez J, Ricquier D, Goubern M, Bouillaud F. Rial E, et al. EMBO J. 1999 Nov 1;18(21):5827-33. doi: 10.1093/emboj/18.21.5827. EMBO J. 1999. PMID: 10545094 Free PMC article. - Mitochondrial proton leak: a role for uncoupling proteins 2 and 3?
Porter RK. Porter RK. Biochim Biophys Acta. 2001 Mar 1;1504(1):120-7. doi: 10.1016/s0005-2728(00)00246-2. Biochim Biophys Acta. 2001. PMID: 11239489 Review. - Mitochondrial uncoupling proteins in human physiology and disease.
Hagen T, Vidal-Puig A. Hagen T, et al. Minerva Med. 2002 Feb;93(1):41-57. Minerva Med. 2002. PMID: 11850613 Review.
Cited by
- UCP2 and pancreatic cancer: conscious uncoupling for therapeutic effect.
Caggiano EG, Taniguchi CM. Caggiano EG, et al. Cancer Metastasis Rev. 2024 Jun;43(2):777-794. doi: 10.1007/s10555-023-10157-4. Epub 2024 Jan 9. Cancer Metastasis Rev. 2024. PMID: 38194152 Free PMC article. Review. - Lysophosphatidic acid modulates CD8 T cell immunosurveillance and metabolism to impair anti-tumor immunity.
Turner JA, Fredrickson MA, D'Antonio M, Katsnelson E, MacBeth M, Van Gulick R, Chimed TS, McCarter M, D'Alessandro A, Robinson WA, Couts KL, Pelanda R, Klarquist J, Tobin RP, Torres RM. Turner JA, et al. Nat Commun. 2023 Jun 3;14(1):3214. doi: 10.1038/s41467-023-38933-4. Nat Commun. 2023. PMID: 37270644 Free PMC article. - Skeletal Muscle Uncoupling Proteins in Mice Models of Obesity.
Križančić Bombek L, Čater M. Križančić Bombek L, et al. Metabolites. 2022 Mar 17;12(3):259. doi: 10.3390/metabo12030259. Metabolites. 2022. PMID: 35323702 Free PMC article. Review. - Erythrophagocytosis by Microglia/Macrophage in Intracerebral Hemorrhage: From Mechanisms to Translation.
Liu J, Zhu Z, Leung GK. Liu J, et al. Front Cell Neurosci. 2022 Feb 14;16:818602. doi: 10.3389/fncel.2022.818602. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35237132 Free PMC article. Review. - Mitochondrial Uncoupling Proteins (UCPs) as Key Modulators of ROS Homeostasis: A Crosstalk between Diabesity and Male Infertility?
Monteiro BS, Freire-Brito L, Carrageta DF, Oliveira PF, Alves MG. Monteiro BS, et al. Antioxidants (Basel). 2021 Oct 30;10(11):1746. doi: 10.3390/antiox10111746. Antioxidants (Basel). 2021. PMID: 34829617 Free PMC article. Review.
References
- Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin M F, Surwit R S, Ricquier D, Warden C H. Nat Genet. 1997;15:269–272. - PubMed
- Klingenberg M. Trends Biochem Sci. 1990;15:108–112. - PubMed
- Rolfe D F, Brand M D. Biosci Rep. 1997;17:9–16. - PubMed
- Skulachev V P. Biochim Biophys Acta. 1998;1363:100–124. - PubMed
- Rolfe D F, Brown G C. Physiol Rev. 1997;77:731–758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases