Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis - PubMed (original) (raw)
Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis
Carol Hamelink et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The adrenal gland is important for homeostatic responses to metabolic stress: hypoglycemia stimulates the splanchnic nerve, epinephrine is released from adrenomedullary chromaffin cells, and compensatory glucogenesis ensues. Acetylcholine is the primary neurotransmitter mediating catecholamine secretion from the adrenal medulla. Accumulating evidence suggests that a secretin-related neuropeptide also may function as a transmitter at the adrenomedullary synapse. Costaining with highly specific antibodies against the secretin-related neuropeptide pituitary adenylate cyclase-activating peptide (PACAP) and the vesicular acetylcholine transporter (VAChT) revealed that PACAP is found in nerve terminals at all mouse adrenomedullary cholinergic synapses. Mice with a targeted deletion of the PACAP gene had otherwise normal cholinergic innervation and morphology of the adrenal medulla, normal adrenal catecholamine and blood glucose levels, and an intact initial catecholamine secretory response to insulin-induced hypoglycemia. However, insulin-induced hypoglycemia was more profound and longer-lasting in PACAP knock-outs, and was associated with a dose-related lethality absent in wild-type mice. Failure of PACAP-deficient mice to adequately counterregulate plasma glucose levels could be accounted for by impaired long-term secretion of epinephrine, secondary to a lack of induction of tyrosine hydroxylase, normally occurring after insulin hypoglycemia in wild-type mice, and a consequent depletion of adrenomedullary epinephrine stores. Thus, PACAP is needed to couple epinephrine biosynthesis to secretion during metabolic stress. PACAP appears to function as an "emergency response" cotransmitter in the sympathoadrenal axis, where the primary secretory response is controlled by a classical neurotransmitter but sustained under paraphysiological conditions by a neuropeptide.
Figures
Figure 1
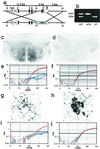
Production of PACAP-deficient mice. (a) Design of the vector for recombination in embryonic stem cells resulting in excision of PACAP-coding sequences from the PACAP gene. Exons 1–5 are labeled; shown in black are the coding regions; the black hashed portion of exon 5 represents the PACAP ORF. _Hin_dIII (H),_Xba_I (X), and _Bam_HI (B) sites are shown. (b) PCR of tail DNA from wild-type, heterozygous, and homozygous F2 mice; wild-type allele 510 bp, knock-out allele 310 bp. (c) PACAP-immunoreactivity (ir) staining of hypothalamus in +/+ F2 mice. (d) PACAP-ir staining of hypothalamus in −/− F2 mice. (e) Q-RT-PCR of hypothalamic cDNA for PACAP from +/+ F2 mice in red and −/− F2 mice in blue. (f) Q-RT-PCR of hypothalamic GAPDH cDNA from +/+ F2 mice in red and −/− F2 mice in blue. (g) Weak PACAP-ir staining of preganglionic sympathetic neurons in intermediolateral column of +/+ mice. (h) VAChT-ir staining of preganglionic sympathetic neurons in adjacent section of intermediolateral column to that stained for PACAP in g. (i) Q-RT-PCR of spinal cord PACAP cDNA from +/+ F2 mice in red and −/− F2 mice in blue. (j) Q-RT-PCR of spinal cord GAPDH cDNA from +/+ F2 mice in red and −/− F2 mice in blue.
Figure 2
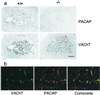
Colocalization of VAChT and PACAP in adrenal medulla. (a) Staining for PACAP and VAChT in wild-type (+/+) and knock-out (−/−) mice. (Scale bar = 150 μm.) (b) Confocal microscopy for VAChT and PACAP costaining of wild-type mouse adrenal medulla. (×425.)
Figure 3
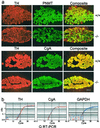
Adrenal morphology. (a) Comparison of TH, PNMT, and CgA staining in wild-type and knock-out adrenal gland. (Scale bar = 150 μm.) (b) Comparison of CgA, TH, and GAPDH cDNA by Q-RT-PCR in wild-type and knock-out mouse adrenal medulla cDNA +/+ F2 mice in red and −/− F2 mice in blue.
Figure 4
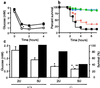
Involvement of PACAP in adrenomedullary responsiveness to metabolic stress. (a) Insulin-induced hypoglycemia, +/+ (■) vs. −/− (○), time course after 5 units/kg insulin. *, P < 0.001 relative to +/+ by one-way ANOVA. (b) Lethality dose–response curve for PACAP −/− mice, 1–10 units/kg insulin, combined male and female mice: blue triangles, 1 unit; green circles, 2 units; red diamonds, 5 units; and black squares, 10 units. The +/+ mice are not shown; survival was 100% at all doses. (c) Bar graphs of plasma glucose (open bars; at 2 h, n = 7–9), and percentage survival (filled bars; at 4 h), +/+ vs. −/−, after administration of insulin at 2 or %5 units/kg. *, P < 0.001 relative to +/+; **, P < 0.05 relative to −/− at 2 units of insulin, by one-way ANOVA with Scheffé's post-hoc test.
Figure 5
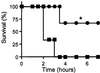
PACAP-38 rescue from insulin-induced lethality in PACAP-deficient mice: ●, PACAP-38 10 nmol; ■, saline. *, P < 0.05 relative to saline by Kaplan–Meier rank test.
Figure 6
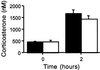
Lack of change in plasma corticosterone induction after insulin (5 units/kg), +/+ (filled bars) vs. −/− (open bars).
Figure 7
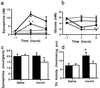
Plasma epinephrine and glucose after insulin shock in PACAP-deficient vs. wild-type mice. (a) Plasma epinephrine levels after insulin: ■, +/+ 2 units of insulin per kg; ●, −/− 2 units of insulin per kg; □, +/+ saline; and ○, −/− saline. *,P < 0.05; **, P < 0.001 relative to +/+ by one-way ANOVA with Scheffé's post-hoc test. (b) Blood glucose levels after insulin: ■, +/+ 2 units of insulin per kg; ●, −/− 2 units of insulin per kg; □, +/+ saline; and ○, −/− saline. *, P < 0.05; **, P < 0.001 relative to +/+ by one-way ANOVA with Scheffé's post-hoc test. (c) Adrenal epinephrine levels 4 h after 2 units of insulin per kg or saline: filled bars, +/+; and open bars, −/−. *,P < 0.05 relative to +/+ by one-way ANOVA with Scheffé's post-hoc test. (d) TH is not induced in PACAP knock-out adrenal medulla 4 h after 2 units of insulin per kg: filled bars, +/+; and open bars, −/−. *,P < 0.05 relative to +/+ saline by one-way ANOVA with Scheffé's post-hoc test.
Similar articles
- PACAP controls adrenomedullary catecholamine secretion and expression of catecholamine biosynthetic enzymes at high splanchnic nerve firing rates characteristic of stress transduction in male mice.
Stroth N, Kuri BA, Mustafa T, Chan SA, Smith CB, Eiden LE. Stroth N, et al. Endocrinology. 2013 Jan;154(1):330-9. doi: 10.1210/en.2012-1829. Epub 2012 Dec 7. Endocrinology. 2013. PMID: 23221599 Free PMC article. - Pituitary adenylate cyclase-activating polypeptide (PACAP) can act as determinant of the tyrosine hydroxylase phenotype of dopaminergic cells during retina development.
Borba JC, Henze IP, Silveira MS, Kubrusly RC, Gardino PF, de Mello MC, Hokoç JN, de Mello FG. Borba JC, et al. Brain Res Dev Brain Res. 2005 May 12;156(2):193-201. doi: 10.1016/j.devbrainres.2005.02.016. Brain Res Dev Brain Res. 2005. PMID: 16099306 - Is PACAP the major neurotransmitter for stress transduction at the adrenomedullary synapse?
Smith CB, Eiden LE. Smith CB, et al. J Mol Neurosci. 2012 Oct;48(2):403-12. doi: 10.1007/s12031-012-9749-x. Epub 2012 May 18. J Mol Neurosci. 2012. PMID: 22610912 Free PMC article. Review.
Cited by
- Inhibition of Ca2+ channels and adrenal catecholamine release by G protein coupled receptors.
Currie KP. Currie KP. Cell Mol Neurobiol. 2010 Nov;30(8):1201-8. doi: 10.1007/s10571-010-9596-7. Cell Mol Neurobiol. 2010. PMID: 21061161 Free PMC article. Review. - Whole genome expression profiling associates activation of unfolded protein response with impaired production and release of epinephrine after recurrent hypoglycemia.
Kim JL, La Gamma EF, Estabrook T, Kudrick N, Nankova BB. Kim JL, et al. PLoS One. 2017 Feb 24;12(2):e0172789. doi: 10.1371/journal.pone.0172789. eCollection 2017. PLoS One. 2017. PMID: 28234964 Free PMC article. - PACAP signaling exerts opposing effects on neuroprotection and neuroinflammation during disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis.
Ringer C, Büning LS, Schäfer MK, Eiden LE, Weihe E, Schütz B. Ringer C, et al. Neurobiol Dis. 2013 Jun;54:32-42. doi: 10.1016/j.nbd.2013.02.010. Epub 2013 Mar 4. Neurobiol Dis. 2013. PMID: 23466699 Free PMC article. - Neuroprotection by endogenous and exogenous PACAP following stroke.
Chen Y, Samal B, Hamelink CR, Xiang CC, Chen Y, Chen M, Vaudry D, Brownstein MJ, Hallenbeck JM, Eiden LE. Chen Y, et al. Regul Pept. 2006 Nov 15;137(1-2):4-19. doi: 10.1016/j.regpep.2006.06.016. Epub 2006 Oct 4. Regul Pept. 2006. PMID: 17027094 Free PMC article. - Pituitary adenylate cyclase-activating polypeptide: Postnatal development in multiple brain stem respiratory-related nuclei in the rat.
Liu Q, Wong-Riley MTT. Liu Q, et al. Respir Physiol Neurobiol. 2019 Jan;259:149-155. doi: 10.1016/j.resp.2018.10.005. Epub 2018 Oct 22. Respir Physiol Neurobiol. 2019. PMID: 30359769 Free PMC article.
References
- Guidotti A, Mao C C, Costa E. In: Frontiers in Catecholamine Research. Usdin E, Snyder S, editors. Oxford: Pergamon; 1973. pp. 231–236.
- Wakade A R. Adv Pharmacol. 1998;42:595–598. - PubMed
- Mueller R A, Thoenen H, Axelrod J. Eur J Pharmacol. 1970;10:51–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases